12. how many milliliters of 0.125 m ba(oh)2(aq) must be used to produce 5.5 moles of water? please balance the equation before solving the problem.
Answers
Ba(OH)2(aq) + 2 H2SO4(aq) → BaSO4(s) + 2 H2O(l)From the equation, we can see that for every mole of Ba(OH)2 used, 2 moles of water are produced. Therefore, to produce 5.5 moles of water, we need to use:
5.5 moles H2O / 2 moles Ba(OH)2 = 2.75 moles Ba(OH)2
To produce 5.5 moles of water, we need to use an equal number of moles of Ba(OH)2. The balanced chemical equation for the reaction is Ba(OH)2(aq) + 2 H2SO4(aq) → BaSO4(s) + 2 H2O(l)
From the equation, we can see that for every mole of Ba(OH)2 used, 2 moles of water are produced. Therefore, to produce 5.5 moles of water, we need to use:
5.5 moles H2O / 2 moles Ba(OH)2 = 2.75 moles Ba(OH)2
The problem gives us the concentration of Ba(OH)2, which is 0.125 M. This means that there are 0.125 moles of Ba(OH)2 in every liter of solution. To find out how many milliliters of 0.125 M Ba(OH)2 we need to use, we first need to convert the number of moles to liters:
2.75 moles Ba(OH)2 × 1 liter / 0.125 moles = 22 liters
Since we need to use milliliters, we can convert liters to milliliters by multiplying by 1000:
22 liters × 1000 ml / 1 liter = 22000 ml
Therefore, we need to use 22000 milliliters, or 22 liters, of 0.125 M Ba(OH)2 to produce 5.5 moles of water.
To know more about moles , visit
https://brainly.com/question/31597231
#SPJ11
To determine the volume of 0.125 M Ba(OH)2(aq) needed, we first need to balance the chemical equation. The balanced equation for the reaction is:
Ba(OH)2(aq) → BaO(s) + 2H2O(l)
According to the balanced equation, 1 mole of Ba(OH)2 produces 2 moles of H2O. Now we can use the given information to find the volume of Ba(OH)2 solution needed:
5.5 moles of H2O × (1 mole of Ba(OH)2 / 2 moles of H2O) = 2.75 moles of Ba(OH)2
Next, use the molarity formula to find the volume in liters:
Volume (L) = moles of solute / molarity
Volume (L) = 2.75 moles of Ba(OH)2 / 0.125 M = 22 L
Convert the volume to milliliters:
22 L × (1000 mL / 1 L) = 22,000 mL
Hence, To produce 5.5 moles of water, you need to use 22,000 mL of 0.125 M Ba(OH)2(aq).
Learn more about equation click here:
https://brainly.com/question/2972832
#SPJ11
Related Questions
i only need help with the ones i didnt fill out I FILLED OUT MOST OF THEM plz help em i need this done as soon as possible

Answers
Answer:
The atomic number and the number of protons are the same so I am only going to list the atomic number. In order from top to bottom.
1
1
11
22
47
9
2
31
31
Why is it that when you heat up a small portion of mac n cheese, it gets warmed up quickly and soon burnt. I heated up my bowl because it was getting cold, only a little in it and it got started poping after 1 second. Why is this?
another fun question, but for real, what does it mean?
Answers
Answer:
well tbh it's just the microwave doing its job heating up your Mac n cheese. But while doing so the Mac n cheese absorbs a lot of heat and then releases it by popping.
Answer:
I don't know
Explanation:
so true though
Why are there 8 elements in the 3rd stage in a periodic table ?
Answers
Answer:
PLEASE MARK ME AS THE BRAINLIEST
Explanation:
According to the 2n2 rule, the maximum number of electrons in the third period = 2 x (3)2 = 18. But, the last shell cannot accommodate more than 8 electrons so, the number of electrons in third period is 8. Hence, the number of elements is also 8.
how to distinguish between aqueous potassium bromide and aqueous potassium iodide TEST AND RESULT
Answers
It is possible to conduct a test to distinguish between potassium chloride and potassium iodide using a silver nitrate solution. Silver nitrate solution and ammonia solution are used in the testing for halide ions.
What happens when potassium iodide and aqueous bromine interact?When bromine-water is introduced to a potassium iodide solution, hydrobromic acid is produced as a byproduct of the oxidation to iodate, which is indicated by a sharp rise in conductivity and a fall in pH.
What is the iodide and bromide ion confirmatory test?The Layer's test is conducted using "carbon disulphide" and "dilute hydrochloric acid." This produces an orange layer when bromide ions are present, and a violet layer when iodide ions are present.
To know more about potassium chloride visit:-
https://brainly.com/question/22528097
#SPJ1
early atomic theories could not explain the ______ of elements
Answers
Explanation:
But I think properties of the element
because at past people were not well informed about the properties of element so later modern periodic table replaces with mendellive periodic table correcting it's bud and mistakes.
what is the ph of 0.80 m khco3
Answers
0.80 m khco3 has a pH of 8.3.
How come urine is acidic?The primary mechanisms by which the kidneys maintain a healthy acid-base balance are sodium reabsorption and the tubular secretion of hydrogen and ammonium ions. When the body retains more sodium and more acid, the urine gets progressively more acidic.
What does food pH mean?The amount of free hydrogen ions contained in a food directly affects its pH level. Food acids produce free hydrogen ions, which are responsible for the unique sour flavor of acidic foods. In this way, we free acidity can be measured by pH.
To know more about pH visit:-
https://brainly.com/question/15289741
#SPJ1
how many milliliters of 0.0200 m ca(oh)₂are required to neutralize 64.1 ml of 0.0300 m hcl?
Answers
48.075 mL
Explanation:
To determine how many milliliters of 0.0200 M Ca(OH)₂ are required to neutralize 64.1 mL of 0.0300 M HCl, you can use the following steps:
1. Write the balanced chemical equation for the reaction between Ca(OH)₂ and HCl:
Ca(OH)₂ + 2HCl → CaCl₂ + 2H₂O
2. Calculate the moles of HCl in the solution:
moles of HCl = (volume of HCl in mL) x (molarity of HCl) / 1000
moles of HCl = (64.1 mL) x (0.0300 M) / 1000 = 0.001923 moles
3. Use the stoichiometry of the balanced equation to find the moles of Ca(OH)₂ required to neutralize the moles of HCl:
moles of Ca(OH)₂ = moles of HCl / 2
moles of Ca(OH)₂ = 0.001923 moles / 2 = 0.0009615 moles
4. Calculate the volume of 0.0200 M Ca(OH)₂ required to neutralize the moles of Ca(OH)₂:
volume of Ca(OH)₂ in mL = (moles of Ca(OH)₂ x 1000) / molarity of Ca(OH)₂
volume of Ca(OH)₂ in mL = (0.0009615 moles x 1000) / 0.0200 M = 48.075 mL
Therefore, 48.075 mL of 0.0200 M Ca(OH)₂ are required to neutralize 64.1 mL of 0.0300 M HCl.
To know more:
https://brainly.com/question/21847159?
#SPJ11
Consider the reaction described by the chemical equation shown. C2H4(g)+H2O(l)⟶C2H5OH(l) ΔH∘rxn=−44.2 kJ C 2 H 4 ( g ) + H 2 O ( l ) ⟶ C 2 H 5 OH ( l ) Δ H rxn ° = − 44.2 k J Use the data from the table of thermodynamic properties to calculate the value of Δ∘rxn Δ S rxn ° at 25.0 ∘C 25.0 ° C .
Δ∘rxn=
Δ S rxn ° =
Δ∘rxn=
Δ G rxn ° =
In which direction is the reaction, as written, spontaneous at 25 ∘C and standard pressure?
Answers
Reverse direction: Reaction is not spontaneous; reactants favored at 25°C.
Spontaneity of chemical reactions at 25°C?To calculate ΔS°rxn, we can use the following equation:
ΔG°rxn = -RTlnK
where R is the gas constant (8.314 J/mol*K), T is the temperature in Kelvin (298 K), K is the equilibrium constant, and ΔG°rxn is the standard Gibbs free energy change for the reaction.
Since ΔG°rxn = ΔH°rxn - TΔS°rxn, we can rearrange the equation as:
ΔS°rxn = (ΔH°rxn - ΔG°rxn) / T
We are given ΔH°rxn as -44.2 kJ, so we need to calculate ΔG°rxn and then use the equation above to find ΔS°rxn.
To calculate ΔG°rxn, we can use the following equation:
ΔG°rxn = ΣnΔG°f(products) - ΣnΔG°f(reactants)
where ΔG°f is the standard Gibbs free energy of formation of each compound, n is the number of moles of each compound in the balanced chemical equation, and the values are given in the table of thermodynamic properties.
For the given reaction, the equation becomes:
ΔG°rxn = [ΔG°f(C2H5OH) - ΔG°f(C2H4) - ΔG°f(H2O)] = [-277.6 - (2*68.3) + (-237.1)] = -39.9 kJ
Now we can substitute the values we have calculated into the equation for ΔS°rxn:
ΔS°rxn = (-44.2 kJ - (-39.9 kJ)) / (298 K) = -0.014 J/K
Since ΔS°rxn is negative, the reaction is not spontaneous at 25°C and standard pressure in the direction as written. However, we can use the Gibbs free energy equation to determine in which direction the reaction will be spontaneous:
ΔG°rxn = -RTlnK
For a spontaneous reaction, ΔG°rxn must be negative, which means that lnK must be negative. Since lnK is negative, K must be less than 1, which means that the reactants are favored at equilibrium. Therefore, the reaction will proceed in the reverse direction as written (i.e. from C2H5OH to C2H4 and H2O) at 25°C and standard pressure.
Learn more about thermodynamic
brainly.com/question/31275352
#SPJ11
A 70-year-old male pays $79. 79 per month for his term life policy while an 80-year-old pays $349. 30 per month. what is the percent of increase in premium costs from age 70 to age 80?
Answers
The percent of increase in premium costs from age 70 to age 80 is approximately 337.84% .To calculate the percent of increase in premium costs .
we need to find the difference between the premium costs at age 80 and age 70, divide that by the premium cost at age 70, and then multiply by 100 to get the percentage increase. So, the difference in premium costs between the 80-year-old and 70-year-old is $349.30 - $79.79 = $269.51. Then, we divide this difference by the premium cost at age 70 $269.51 ÷ $79.79 = 3.3794 ,Finally, we multiply this result by 100 to get the percentage increase ,3.3794 x 100% = 337.84% .
To calculate the percent increase, follow these steps ,Step 1: Determine the difference in premium costs between the two ages $349.30 (cost for an 80-year-old) - $79.79 (cost for a 70-year-old) = $269.51 ,Step 2: Divide the difference by the original cost (cost for a 70-year-old). $269.51 / $79.79 = 3.3761 Step 3: Multiply the result by 100 to get the percentage. 3.3761 x 100 = 337.61% ,So, there is a 337.61% increase in premium costs from age 70 to age 80.
To know more about premium costs visit :
https://brainly.com/question/14414537
#SPJ11
the sds for 1-octanol is provided here. (links to an external site.) is 1-octanol a combustible liquid?
Answers
True. 1-octanol is a combustible liquid with a flashpoint of 86°C and an auto-ignition temperature of 258°C, according to the provided SDS.
The SDS (Safety Data Sheet) for 1-octanol indicates that it is a combustible liquid. According to the SDS, 1-octanol has a flashpoint of 86°C (187°F) and an auto-ignition temperature of 258°C (496°F). These values suggest that 1-octanol can easily ignite in the presence of an ignition source and may burn at relatively low temperatures. Additionally, the SDS provides information on the fire and explosion hazards associated with 1-octanol and recommends appropriate handling procedures and precautions to minimize the risk of fire or explosion. Therefore, it is important to handle 1-octanol with care and follow appropriate safety protocols when working with this substance.
To learn more about combustible liquid, refer:
https://brainly.com/question/28222891
#SPJ4
The complete question is:
the SDS for 1-octanol is provided here. (links to an external site.) is 1-octanol a combustible liquid? True or False.
chlorine compounds remain stable and effective in the presence of excess organic matter. T/F
Answers
True. Chlorine compounds are effective disinfectants and can remain stable even in the presence of excess organic matter. However, it is important to note that the effectiveness may be reduced over time as the content of loaded matter increases.
Chlorine compounds, specifically content loaded chlorine compounds, can remain stable and effective in the presence of excess organic matter. These compounds maintain their disinfecting and sanitizing properties even when exposed to large amounts of organic matter.When present with organic materials, chlorine compounds may react and lose their effectiveness. As a result, the ability to disinfect may be diminished, which could lead to the persistence of dangerous diseases. Water treatment facilities frequently use pre-treatment procedures or alternate disinfection techniques that are less prone to interference from organic matter to address this issue.
To know more about Chlorine compounds Visit:
https://brainly.com/question/6349911
#SPJ11
Which statement below best describes a catalyst?
Question 3 options:
An item that can slow reactions rates
A molecule that is consumed in a chemical reaction
An item that can increase reaction rates
An item that increases the concentration of reactions
Answers
As the volume of a gas increases, its density
(Assume all other factors are held constant).

Answers
Answer:
C) Decreases
Explanation:
Density and volume are inversely related to each other.
Hope this helped :)
why carbon nanotubes are ao strong?
Answers
Regina has avoided working on her term paper all semester, and now she has only one week to write it. She identifies her goal and hastily writes it down: I will make an A on my English literature term paper that is due next week. Then, she starts breaking her goal into specific daily tasks. Where is the flaw in Regina’s plan?
She hasn’t broken her big goal into small enough pieces.
Her time frame is not realistic.
Her goal is not specific enough.
She has not written her goal enough times.
Answers
The flaw in Regina’s plan is that the time frame is unrealistic (option B).
What is goal setting?Goal setting involves the development of an action plan designed in order to motivate and guide a person or group toward a goal, which is a result that one is attempting to achieve.
Effective goal setting lets you measure progress, overcome procrastination and visualize your dreams. However, setting goals is said to be effective when it is done in an adequate time frame.
According to this question, Regina identifies her goal and hastily writes it down as follows: I will make an A on my English literature term paper that is due next week.
This goal, although is great, is unlikely to be met because of the time frame. One cannot get an excellent result in something that lacks adequate preparation, which includes adequate time.
Learn more about goal setting at: https://brainly.com/question/1705973
#SPJ1
What is the volume of the water?
0 points
Captionless Image

Answers
Answer:
the value of water is
6000
Answer:
600 or 6000 I'm not sure
Which of the following would be a part of an environment engineer's job description ?
Answers
Answer:
where is the options
Explanation:
give options
Use Le Châtelier’s principle to predict what will happen to the following equilibrium if the volume is decreased.
2NH3(g) ↔ N2(g) + 3H2(g)
Answers
Answer:
The reverse reaction is favoured
Explanation:
According to the principle; when a constraint such as a change in concentration, pressure, temperature etc is imposed on a reaction system in equilibrium, the equilibrium position will shift in such a direction as to annul the constraint.
In this case, the constraint is a change in volume. If the volume is decreased, the reverse reaction (leading to less total volume) is favoured.
The forward reaction leads to four volumes while the reverse reaction leads to only two volumes. Decrease in volume favours the direction in which the total volume is lower.
which chemical is used in both water purification and sewage treatment to provide long-term disinfection?
Answers
The chemical commonly used in both water purification and sewage treatment to provide long-term disinfection is chlorine.
Chlorine compounds, such as chlorine gas (Cl2), sodium hypochlorite (NaClO), or calcium hypochlorite (Ca(ClO)2), are added to water or wastewater to kill or inactivate harmful microorganisms, including bacteria, viruses, and parasites. Chlorine acts as a powerful disinfectant by disrupting the cellular structures and metabolic processes of microorganisms, preventing their reproduction and causing their death.
In water purification, chlorine is added to treat drinking water sources and ensure its safety for consumption. It helps to eliminate pathogens and protect against waterborne diseases. In sewage treatment, chlorine is used to disinfect wastewater before it is discharged back into the environment. This step helps to prevent the spread of disease-causing organisms and protect public health.
However, it is important to note that the use of chlorine in water treatment requires careful control and monitoring to ensure proper dosage and minimize potential risks associated with disinfection by-products.
To know more about chemical click this link -
brainly.com/question/29240183
#SPJ11
how much electrical energy (in kilowatt-hours) would a 60.0W light bulb use in 60.0 days ifvleft steadily
Answers
Answer:
60w x 24 = 1440wh per day
1440 x 60 = 86400 WATTS HOUR FOR 60 DAYS
Explanation:
In short.
86400/1000 = 86.4kwh
brainiest me :> THANK YOU!
Answer:
86.4kwh
Explanation:
I don’t even know what this is. Please help

Answers
FeCl2
FeCl3
GaCl3
AgCl
PbCl4
Balance the chemical equation. Values may be used more than once.
Ga + H2SO4 ⇒ Gaz(SO4)3 + H2
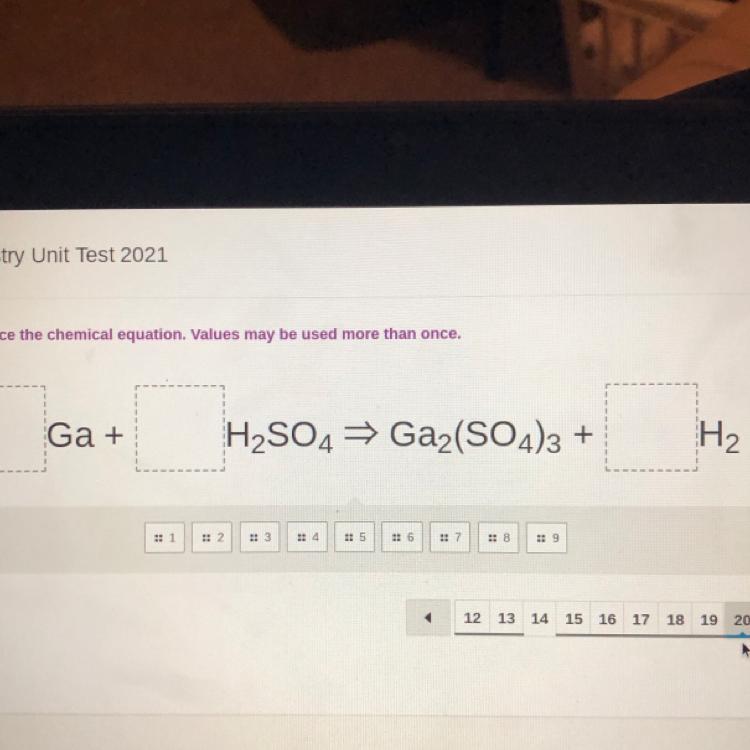
Answers
\(\\ \sf\longmapsto Ga+H_2SO_4\longrightarrow Ga_2(SO_4)_3+H_2\)
Balanced equation:-
\(\\ \sf\longmapsto 2Ga+3H_2SO_4\longrightarrow Ga_2(SO_4)_3+3H_2\)
On reactant side
Ga=2H=6SO_4=3On products side
Ga=2H=6SO_4=3Hence balanced
in each reaction box, place the best reagent and conditions from the list. you are currently in a labeling module. turn off browse mode or quick nav, tab to items, space or enter to pick up, tab to move, space or enter to drop.benzene is converted to bupropion, which has the commercial name zyban. this is used to help people quit smoking. four reagents are needed. the structure of zyban is a benzene ring with a ketone on carbon 1 and a chloride on carbon 3. the ketone is bonded to c h (c h 3) n h c (c h 3) 3. answer bank
Answers
The best reagents used are acetyl chloride, LiAlH4, thionyl chloride and trimethylamine.
The best reagent and conditions from the list that are used to convert benzene to bupropion, which is used to help people quit smoking, are as follows :
Reaction 1: A Friedel-Crafts acylation reaction is used to introduce the ketone into the benzene ring. Therefore, the reagent required is acetyl chloride (CH3COCl) and the catalyst required is aluminum chloride (AlCl3). Conditions are anhydrous and at a low temperature.
Reaction 2: The best reagent to use in the reduction of the ketone is lithium aluminum hydride (LiAlH4). The reaction requires anhydrous and ether solvent conditions.
Reaction 3: A reaction with chlorination agent is used to introduce a chlorine atom into position 3 of the benzene ring. Therefore, the reagent used is thionyl chloride (SOCl2). The reaction requires pyridine solvent conditions.
Reaction 4: The final reaction is a substitution reaction that involves a nucleophilic attack by an amine on a benzyl chloride. The reagent used in this reaction is NH2(CH3)3, which is also known as trimethylamine. The reaction requires anhydrous conditions and a polar aprotic solvent such as dimethylformamide (DMF).
Thus, the best reagents are CH3COCl, LiAlH4, SOCl2, NH2(CH3)3.
To learn more about benzene :
https://brainly.com/question/29585681
#SPJ11
A gas has a volume of 13.4 L at 17C. What is the volume of the gas at standard temperature?
Answers
Answer:
This law states that the volume and temperature of a gas have a direct relationship: As temperature increases, volume increases, when pressure is held constant. Heating a gas increases the kinetic energy of the particles, causing the gas to expand.
Explanation:
Considering the Charles's law and STP conditions, the volume of the gas at standard temperature is 12.61 L.
Charles's lawCharles's law establishes the relationship between the volume and temperature of a gas sample at constant pressure.
This law says that for a given sum of gas at constant pressure, as the temperature increases, the volume of the gas increases and as the temperature decreases, the volume of the gas decreases. That is, the volume is directly proportional to the temperature of the gas.
Mathematically, Charles's law states that the ratio between volume and temperature will always have the same value:
\(\frac{V}{T} =k\)
Considering an initial state 1 and a final state 2, it is fulfilled:
\(\frac{V1}{T1} =\frac{V2}{T2}\)
Definition of STP conditionThe STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C (or 273 K) are used and are reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
Volume of the gas at standard temperatureIn this case, you know:
V1= 13.4 LT1= 17 C= 290 K (being 0 C= 273 K)V2= ?T2= 0 C= 273 K (at STP)Replacing in the definition of Charles's law:
\(\frac{13.4 L}{290 K} =\frac{V2}{273 K}\)
Solving:
\(V2= 273 K\frac{13.4 L}{290 K}\)
V2= 12.61 L
Finally, the volume of the gas at standard temperature is 12.61 L.
Learn more about
Charles's law:
https://brainly.com/question/4147359
STP conditions:
brainly.com/question/26364483
brainly.com/question/8846039
brainly.com/question/1186356
#SPJ2
In what way can motion help in our daily lives?
Answers
Motion can help us in lot of ways.
Motion ensures that blood flows into our bodies.
It enhances the person's mood, ensures a healthy body, healthier bones and healthier lifestyle.
4. Calculate the heat energy needed to heat 25.0 g of water from 13.0°C to 18.0°C. Use chart above for specific heat of water. Use units and show all work. ΔΗ=mcΔΤ AH=( XOXO AH= I 5. Calculate the heat energy needed to heat 25.0 g of aluminum from 13.0°C to 18.0°C. Use chart above for specific heat of water. Use units and show all work. ΔΗ=mcΔΤ AH=( 00 AH= 6. Calculate the heat energy needed to heat 45.0 g of copper from 23.0°C to 30.0°C. Use chart above for specific heat of water. Use units and show all work. Ah-meAT
Answers
Answer:
f
Explanation:
The electronegativitie of the period-3 element are lited on the tranparency. Calculate the
electronegativity difference for the following pair of bonded period-3 atom. D. Si and Cl _______________________
b. Mg and S_______________________
e. Si and S ________________________
c. Al and P ______________________
Answers
The electronegativitie of the period-3 element are lited on the tranparency.. Si and Cl is 0.7, . Mg and S is 1.3, Si and S is 1.7, Al and P is 1.6.
The electronegativity of the atoms that make up the bond determines whether it is nonpolar or polar covalent. An atom's propensity to draw electrons (or electron density) in its direction is measured by its electronegativity. It controls how the shared electrons between the two atoms in a bond are distributed. The more an atom's electronegativity, the more strongly the electrons in its bonds are drawn to it. In a polar covalent bond, electrons are pushed in the direction of the more electronegative atom; as a result, the atom with the partial negative charge is the more electronegative atom. The electron distribution becomes increasingly polarised and the atoms' partial charges increase with increasing electronegativity differences.
Learn more about electronegativity here:
https://brainly.com/question/29810769
#SPJ4
Help me asap, due today

Answers
1. The equation is not balanced. The balanced equation is \(4NH_3 + 5O_2 --- > 4NO +6 H_2O\)
2. The equation is balanced.
3. The equation is not balanced. The balanced version is \(2H_2O --- > 2H_2 + O_2\)
Balancing chemical equationsA balanced chemical equation usually has the same number of atoms of different elements in the reactants and the products, even though the forms of the atoms might have changed.
Consider the first equation: \(NH_3 + O_2 --- > NO + H_2O\)
The number of hydrogen atoms is not balanced. Thus, the equation that shows balanced atoms of different elements would be: \(4NH_3 + 5O_2 --- > 4NO +6 H_2O\)
Consider the second equation: \(N_2 + 3H_2 -- > 2NH_3\)
There are 2 atoms of nitrogen in the reactants and there are also 2 in the product. The number of hydrogen atoms is 6 in the reactants and 6 in the products. Thus, it is a balanced equation.
Consider the third equation: \(2H_2O --- > H_2 + O_2\)
There are 4 hydrogen atoms in the reactant and only 2 in the products. The balanced equation would be: \(2H_2O --- > 2H_2 + O_2\)
More on balancing chemical equations can be found here: https://brainly.com/question/28294176
#SPJ1
You work in the special effects department of a movie studio. You are
currently working on a superhero movie where the hero is very strong
and can punch through metal. For the next scene you need to replace a
6 inch by 6 inch square of a metal wall with a different material that will
crumble when the actor hits it. What could you use?
A. You could use Carbon(C)
B. You could use Potassium (k)
C. You could use Titanium (T)
D. You could use Manganese (Mn)
Answers
Answer:
The correct option is;
D. Manganese (Mn)
Explanation:
Manganese is very brittle, hard, iron like silvery-gray metal, that is difficult to melt. In air, Manganese slowly disintegrate in a similar manner to iron rusting in water
Manganese and iron have similar chemical and physical properties however manganese is more harder and more brittle than iron
A brittle material is one that easily breaks without deforming elastically
Therefore, manganese, due to its very iron like appearance and brittle nature will be suitable to replace the metal wall and crumble easily when the actor hits it.
When tow forces act in the same direction, they are __________ together
Answers
Answer:
Added
Explanation:
Force is a vector quantity. It follows vector law of addition.
If two forces act in the same direction, the angle between the two force vectors become zero and thus the resultant force turns out to be the algebraic addition of the applied individual forces.
Feq = √(F1²+F2²+2F1.F2.cos0) = √(F1+F2)² = F1+F2.