4.2 mol of oxygen and 4.0 mol of no are introduced to an evacuated 0.50 l reaction vessel. at a specific temperature, the equilibrium 2no(g) o2(g) 2no2(g) is reached when [no]
Answers
Value of Kc for the reaction is 3.9.
Why is the equilibrium constant important?The quantity of reactant to product in a chemical reaction may be compared to derive the equilibrium constant, which is used to predict chemical behaviour. Rate of the reaction in forward direction equals Rate of the reverse reaction at equilibrium.
How to determine Kc for the reaction.Given, moles of oxygen = 4.2 mol
moles of NO = 4.0 mol
Now, Molarity of O2 = moles/volume = 4.2/0.5
Molarity of O2 = 8.4M
Molarity of NO = moles/volume = 4/0.5
Molarity of NO = 8M
The equilibrium reaction is as follows
2NO + O₂ ⇄ 2NO₂
Initially conc. 8.4 8.0 0
At equilibrium (8.4-2x) (8.0-x) 2x
the Kc expression is,
Kc = (NO₂)² / (NO)²× O₂
Kc = (2x)²/ (8.4-2x)² (8.0-x)
Concentration of NO at equilibrium = 1.6 M = (8.4-2x)
8.4 - 2x = 1.6
x = 3.4
After putting the value of x in the Kc equation,
Kc = (2×3.4)²/ (8.4-2×3.4)² (8.0-3.4)
Kc = 3.9
Value of Kc for the reaction is 3.9.
To know more about equilibrium constant visit
brainly.com/question/15118952
#SPJ4
The complete question is;
Suppose 4.2 mol of oxygen and 4.0 mol of NO are introduced to an evacuated 0.50-L reaction vessel. At a specific temperature, the equilibrium 2NO(g) + O2(g) Picture 2NO2(g) is reached when [NO] = 1.6 M. Calculate Kc for the reaction at this temperature.
Related Questions
frequently used in shampoos. the detergent sodium dodecyl sulfate (sds) denatures proteins. suggest how sds destroys protein structure.
Answers
Sodium dodecyl sulfate (SDS), a commonly used detergent in shampoos, can disrupt protein structure through a process called denaturation.
Denaturation refers to the alteration of a protein's native structure, leading to loss of its functional properties. Here's how SDS can contribute to protein denaturation:
Hydrophobic interactions: SDS molecules have a hydrophobic tail that can interact with the hydrophobic regions of proteins. Proteins have hydrophobic amino acid residues buried within their interior, contributing to their structural stability.
When SDS interacts with these hydrophobic regions, it can disrupt the hydrophobic interactions holding the protein's structure together, causing unfolding and denaturation.
Disruption of hydrogen bonds: Proteins rely on hydrogen bonds for their secondary and tertiary structures. SDS can disrupt these hydrogen bonds by competitively binding to the polar regions of the protein, weakening the interactions.
As a result, the protein's folded structure can unravel.
Electrostatic interactions: SDS is an anionic detergent, meaning it carries a negative charge. It can interact with positively charged regions of proteins through electrostatic interactions. This interaction can disrupt the protein's stability by altering the balance of charges and affecting its overall structure.
Denaturation by stripping water molecules: SDS has a strong affinity for water molecules and can solubilize hydrophobic substances.
In the presence of SDS, water molecules that surround the protein can be displaced, leading to protein unfolding and denaturation.
Overall, the interactions of SDS with proteins can disrupt their hydrophobic interactions, hydrogen bonds, electrostatic interactions, and water interactions, resulting in the denaturation and loss of protein structure and function.
Learn more about denaturation from the given link
https://brainly.in/question/1168480
#SPJ11
And ironic bonds what happens to electrons? No

Answers
Answer:
metals donate electrons to nonsmetals
What different methods can be used to create electrodes on a
Borosilicatglass wafer? What additional step has to be performed,
if you want to use a silicon wafer instead?
Answers
The different methods that can be used to create electrodes on a Borosilicate wafer are of standard and thin wall configurations.
The use of standard with filament configuration and thin wall configurations comes in different barrel sizes of one, two, three, five, and seven barrels. The capillaries that line the wall of the glass have the electrodes with the association, if needed, a wire that runs along to the record.
The thin wall single barrel configurations may be fitted with two electrodes. They do not use filings like with the standard configurations.
In order to use a silicon wafer, the additional step that is done is doping. Doping is the introduction of some impurities to the semiconductors to make them more electrically active.
To learn more about Electrodes on Borosilicate glass:
brainly.com/question/31859109
#SPJ4
Name the first 3 alkali metals in group 1
Answers
Answer:
Explanation:
Francium, Sodium, Potassium
Click on the image that most accurately represents a pure
compound.
Corre
Incor
Answers
The nucleus of the eukaryotic cell is important because it
A. Controls the passing of substances through the cell membrane
B. Its rigidity controls the passing of substances through the cell wall
C. Contains DNA and controls the cell
Answers
For this question I think the answer is C.
It is the location of a cell's DNA
Which means it controls the cells
Calculate the relative formula mass of sodium hydroxide (NaOH. Na = 23, O = 16, H = 1)
Answers
Answer:
40g/mol
Explanation:
Na + O + H
23 + 16 + 1
= 40g/mol
what is the formula for caculating time
Answers
Answer: Time = Distance ÷ Speed
how could the following compounds be synthesized from acetylene? compound a is ch3ch2ch2cch, with a triple bond between the fourth (from left to right) and the fifth carbons. compound b is ch3chch2, with a double bond between the second (from left to right) and the third carbons. compound c has a structure of a cc double bond in its structure, with a ch3 group attached to both carbons above and an h atom attached to both carbons below the double bond. compound d is ch3ch2ch2ch2ch, with an o atom double-bonded to the fifth (from left to right) carbon. compound e is ch3chch3, with a br atom attached to the second carbon. compound e is ch3cch3, with two cl atoms attached to the second carbon.
Answers
O
II
CH₃-C-CH₃ The compound that is produced from acetylene.
what is acetylene ?
Acetylene (systematic name: ethyne) is the chemical compound with the formula C2H2 and structure H−C≡C−H. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities such as divinyl sulfide and phosphine.
As an alkyne, acetylene is unsaturated because its two carbon atoms are bonded together in a triple bond. The carbon–carbon triple bond places all four atoms in the same straight line, with CCH bond angles of 180°
To learn more about acetylene follow the given link: https://brainly.com/question/13993072
#SPJ4
What does a reaction energy diagram represents
Answers
Answer:
The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a reaction progress curve. A potential energy diagram shows the change in potential energy of a system as reactants are converted into products.
Explanation:
Answer:
The answer is the changes in energy during a reaction. Have a good day.:)
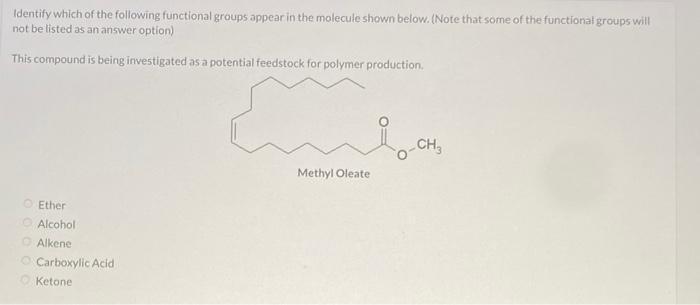
identify which of the following functional groups appear in the molecule shown below. (note that some of the functional groups will not be listed as an answer option)
Answers
The functional groups that we have in the compound are ketone and alkene. Options C and E
What is a functional group?
A functional group is a particular set of atoms in a molecule that are in charge of the molecule's distinctive chemical processes and characteristics. The behavior and functionality of a molecule in chemical processes are determined by a reactive component of the molecule.
The common atoms found in functional groups are carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorus. They can be recognized by their unique atom arrangement and bonding structure inside a molecule.
Learn more about functional group:https://brainly.com/question/1356508
#SPJ4
How do I calculate the number of moles of AI2O3

Answers
The number of mole of Al₂O₃ produced when 0.60 mole of Fe is produced is 0.2 mole
How do I determine the number of mole of Al₂O₃ produced?We'll begin by obtaining the mole of Al that reacted to produce 0.6 mole of Fe. Details below:
2Al + 3FeO -> 3Fe + Al₂O₃
From the balanced equation above,
3 moles of Fe were produced from 2 moles of Al
Therefore,
0.6 mole of Fe will be produce from = (0.6 × 2) / 3 = 0.4 mole of Al
With the above information, we can determine the number of moles of Al₂O₃ produced. This can be obtained as follow:
From the balanced equation above,
2 moles of Al reacted to produce 1 mole of Al₂O₃
Therefore,
0.4 moles of Al will react to produce = (0.4 × 1) / 2 = 0.2 mole of Al₂O₃
Thus, number of mole of Al₂O₃ produced is 0.2 mole
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
please help me im so counfused

Answers
The slopes and the y-intercepts are
2/5 and (0 , - 2). S and O. 3/2 and (0 , - 3). N and K. 1 and (0 , - 6). M and B.- 1 and (0 , - 4). A and Q.3 and (0 , 0). P and J. - 1/2 and (0 , 3). F and G. 5/2 and (0 , - 5). E and L.2/3 and (0 , 1). H and D.The formula for calculating the slope of a line from the two coordinates that the line passes through A (x₁ , y₁) and B (x₂ , y₂)
m = (y₂ - y₁) ÷ (x₂ - x₁)
The y-intercept will occur if the value of x = 0.
1. Pick the first and the last coordinates (- 5 , - 4) and (10 , 2)
Slopem = (y₂ - y₁) ÷ (x₂ - x₁) = (2 - (- 4)) ÷ (10 - (- 5)) = (2 + 4) ÷ (10 + 5)
m = 6 ÷ 15 = 6/15 = 2/5
Sy-intercept
From the table it shows (0 , - 2)
O
2. (2 , 0) and (8 , 9)
Slopem = (y₂ - y₁) ÷ (x₂ - x₁) = (9 - 0) ÷ (8 - 2) = 9 ÷ 6 = 9/6 = 3/2
Ny-intercept (2 , 0) and (0 , y)
m = (y₂ - y₁) ÷ (x₂ - x₁)
3/2 = (y - 0) ÷ (0 - 2)
3/2 = y/- 2
2y = - 6
y = - 3
(0 , - 3)
K
3. (1 , - 5) and (5 , - 1)
Slopem = (y₂ - y₁) ÷ (x₂ - x₁) = (- 1 + 5) ÷ (5 - 1) = 4 ÷ 4 = 1
My-intercept (1 , - 5) and (0 , y)
m = (y₂ - y₁) ÷ (x₂ - x₁)
1 = (y + 5) ÷ (0 - 1)
1 = (y + 5) /- 1
y + 5 = - 1
y = - 1 - 5
y = - 6
(0 , - 6)
B
4. (- 1 , 5) and (5 , - 1)
Slopem = (y₂ - y₁) ÷ (x₂ - x₁) = (- 1 - 5) ÷ (5 + 1) = - 6 ÷ 6 = - 1
Ay-intercept (- 1 , 5) and (0 , y)
m = (y₂ - y₁) ÷ (x₂ - x₁)
- 1 = (y - 5) ÷ (0 + 1)
- 1 = (y - 5) /1
y - 5 = - 1
y = - 1 + 5
y = 4
(0 , 4)
Q
5. (1 , 3) and (4 , 12)
Slopem = (y₂ - y₁) ÷ (x₂ - x₁) = (12 - 3) ÷ (4 - 1) = 9 ÷ 3 = 3
Py-intercept (1 , 3) and (0 , y)
m = (y₂ - y₁) ÷ (x₂ - x₁)
3 = (y - 3) ÷ (0 - 1)
3 = (y - 3)/- 1
y - 3 = - 3
y = - 3 + 3
y = 0
(0 , 0)
J
6. (- 6 , 6) and (0 , 3)
Slopem = (y₂ - y₁) ÷ (x₂ - x₁) = (3 - 6) ÷ (0 + 6) = - 3 ÷ 6 = - 1/2
Fy-intercept
From the table it shows (0 , 3)
G
7. (0 , - 5) and (6 , 10)
Slopem = (y₂ - y₁) ÷ (x₂ - x₁) = (10 + 5) ÷ (6 - 0) = 15 ÷ 6 = 15/6 = 5/2
Ey-intercept
From the table it shows (0 , - 5)
L
8. (- 3 , - 1) and (6 , 5)
Slopem = (y₂ - y₁) ÷ (x₂ - x₁) = (5 + 1) ÷ (6 + 3) = 6 ÷ 9 = 6/9 = 2/3
Hy-intercept
From the table it shows (0 , 1)
D
Learn more about slope here: https://brainly.com/question/18439055
#SPJ1

how are the earthquakes distributed on the map
Answers
The earthquakes distributed on the map on the fault lines at edge of tectonic plates.
Tectonic plate boundaries, or along fault lines, are where earthquakes are most likely to occur. On a map showing tectonic plates, earthquakes will be distributed along the lines on the map. The earthquake epicenter locations shown on the map are not dispersed around the earth's surface at random. The majority of earthquake epicenters are confined to a small area. Most earthquake epicenters, including those in the western parts of North and South America, are found at the edges of certain continents. The Pacific Plate is likewise surrounded by earthquakes. Along the Himalayan mountain range, which is over India, there are also frequent earthquakes. Additionally, all regions with active volcanoes have an earthquake epicenter, but not all regions with earthquake epicenters have active volcanoes, if you try to compare the locations of earthquake epicenters and active volcanoes.To learn more about earthquakes visit:
https://brainly.com/question/1176195
#SPJ9
Gay-lussacs law explanation?
Answers
PLS MARK BRAINLIEST
I need one more to rank up!!
Answer:
:)
Explanation:
Gay-Lussac's Law states that the pressure of a given mass of gas varies directly with the absolute temperature of the gas, when the volume is kept constant.
Calculate the bond order for one carbon-carbon bond in the benzene molecule, taking σ and π bonding into consideration?
Answers
the bond order for one carbon-carbon bond in the benzene molecule, taking σ and π bonding into consideration will be 1.5.
In the benzene molecule, there are 6 carbon atoms arranged in a hexagonal ring, with alternating single and double bonds. Each carbon atom is covalently bonded to two other carbon atoms, with one single bond and one double bond.
To calculate the bond order for one carbon-carbon bond in benzene, we need to take into account both the σ (sigma) and π (pi) bonding. The σ bond is formed by the overlap of two atomic orbitals along the internuclear axis, while the π bond is formed by the overlap of two atomic orbitals perpendicular to the internuclear axis.
In the case of benzene, each carbon-carbon bond has one σ bond and one π bond. The total bond order for each bond can be calculated as the sum of the bond orders for the σ and π bonds. The bond order for a σ bond is 1, while the bond order for a π bond is 0.5.
Therefore, the bond order for one carbon-carbon bond in the benzene molecule is:
Bond order = (σ bond order) + (π bond order) = 1 + 0.5 = 1.5
So the bond order for one carbon-carbon bond in the benzene molecule is 1.5.
Learn more about carbon here:
https://brainly.com/question/3049557
#SPJ4
if 10.0 ml of blood plasma has a mass of 10.279 g and contains 0.870 g of protein, what is the mass/mass percent concentration of protein in the blood plasma? 8.70% 8.46% 32.1% 97.3% 0.870%
Answers
The mass/mass percent concentration of protein in the blood plasma is 8.46%.
The mass/mass percent concentration of protein in the blood plasma can be calculated by dividing the mass of the protein by the mass of the blood plasma and multiplying by 100.
Given that the mass of the blood plasma is 10.279 g and the mass of the protein is 0.870 g, we can calculate the mass/mass percent concentration of protein as follows:
(0.870 g protein / 10.279 g blood plasma) * 100 = 8.46%
Therefore, the mass/mass percent concentration of protein in the blood plasma is 8.46%.
To know more about protein visit:-
https://brainly.com/question/33861617
#SPJ11
Help I’m stuck on this question :
Write equations that show the processes that describe the
first, second, and third ionization energies of an aluminum
atom. Which process would require the least amount of
energy?
If any of you brainiacs could answer, that would be great!
Answers
Answer:
The first ionization energy is the energy it takes to remove an electron from a neutral atom.
hope it is helpful :)
Which of the following represents the integrated rate law for a zeroth-order reaction?
A) ln([A]t/[A]o) = - kt
B) (1/[A]t) - (1/[A]o] = kt
C) [A]t - [A]o = - kt
D) k = Ae(-Ea/RT)
E) ln(k2/k1) = Ea/R (1/T ) + lnA
Answers
The integrated rate law for a zeroth-order reaction is represented by option C) [A]t - [A]o = - kt.
The integrated rate law describes the relationship between the concentration of a reactant and the time for a specific order of the reaction. In the case of a zeroth-order reaction, the rate of the reaction is independent of the concentration of the reactant.
Option C) [A]t - [A]o = - kt represents the integrated rate law for a zeroth-order reaction. In this equation, [A]t represents the concentration of the reactant at a given time, [A]o represents the initial concentration of the reactant, k is the rate constant of the reaction, and t is the time.
The equation shows that the change in concentration of the reactant ([A]t - [A]o) is directly proportional to the elapsed time (t) and the rate constant (k). The negative sign indicates that the concentration decreases over time in a zeroth-order reaction.
Learn more about the zeroth-order reaction here: brainly.com/question/30512199
#SPJ11
Which of these statements is false? The least reactive elements are in the far right column. Elements at the bottom of a column have more protons than elements at the top of a column. Elements on the left side of a row always have higher atomic mass than elements on the right side of that row. Elements along the steplike line have some properties of metals and some properties of nonmetals.
Answers
Answer:
Elements on the left side of a row always have higher atomic mass than elements on the right side of that row.
Explanation:
the answer I have above should be correct because it’s false so it’s the right answer.
What volume container is needed to hold 4.0 grams CO gas at 277 K and 522 torr? Assume ideal gas behavior. Report your answer in Liters with one decimal place.
Answers
Assuming ideal gas behavior, 4.61 liters volume container is needed to hold 4.0 grams CO gas at 277 K and 522 torr.
To determine the volume container needed to hold 4.0 grams of CO gas at 277 K and 522 torr, we need to use the ideal gas law equation PV=nRT. We can rearrange this equation to solve for the volume V, which is the unknown in this case.
First, we need to convert the given mass of CO gas to moles by dividing it by the molar mass of CO, which is 28.01 g/mol.
n = 4.0 g / 28.01 g/mol = 0.1428 mol
Next, we can plug in the given values for temperature T and pressure P, converting pressure from torr to atm:
P = 522 torr / 760 torr/atm = 0.6868 atm
T = 277 K
Finally, we can solve for V:
V = nRT/P = (0.1428 mol)(0.0821 L·atm/mol·K)(277 K)/(0.6868 atm)
V = 4.61 L
Therefore, a container with a volume of 4.61 liters is needed to hold 4.0 grams of CO gas at 277 K and 522 torr, assuming ideal gas behavior.
For more such questions on ideal gas, click on:
https://brainly.com/question/30567855
#SPJ11
CO2(g) +H2(g)------>CO(g) + H2O(/)
What is being oxidized?
Carbon
Carbon Dioxide
Oxygen
Hydrogen
Answers
Answer:
Srry its so hard
Explanation:
for a hydorgen atom, which electronic tranisiton would result in the emisson of a photon with the highest energy
Answers
The electronic transition that would result in the emission of a photon with the highest energy for a hydrogen atom is the transition from the third energy level (n=3) to the first energy level (n=1).
This transition is also known as the Lyman-alpha transition and corresponds to an ultraviolet photon with a wavelength of 121.6 nm. At this energy level, the electron is transitioning from a higher energy level to a lower energy level, resulting in the emission of energy in the form of a photon. The energy of the photon is directly proportional to the frequency and inversely proportional to the wavelength of the emitted radiation.
To learn more about photon, click here:
https://brainly.com/question/20912241
#SPJ11
A nugget of gold is placed in a graduated cylinder that contains 80 mL of water. The water level rises to 225 mL after the nugget is added to the cylinder. What is the volume of the gold nugget?
Answers
Answer:
145 mLExplanation:
The volume of the nugget can be found by using the formula
volume of object = final volume of water - initial volume of water
From the question
final volume of water = 225 mL
initial volume = 80 mL
We have
volume = 225 - 80 = 145 mL
We have the final answer as
145 mLHope this helps you
What is the volume of the fluid in the graduated cylinder measured to the correct degree of precision?
Use this media to help you complete the question.
43.4 mL
44.0 mL
43.42 mL
44.10 mL
Answers
Answer:
44.10
Explanation:
got it right on odessyware
12. The atomic number of an element represents the number of ______________ in the nucleus?
Answers
The number of protons in a nucleus is called the atomic number.
Answer:
atoms in the nucleus
Explanation:
if you read very well
Step1: Cl3 Cl2 +Cl Step2: Cl3+Cl 2Cl2 Which is the activated omplex for step 2?
Answers
Answer:
The answer is "\(\bold{Cl_4}\)"
Explanation:
Please find the complete question in the attachment file.
In this question, it uses the Chemical equations, which is also known as the order to comply with the Law on Mass Conservation, substances must be balanced wherever volume cannot be generated or lost. This should be reacted that the reaction weight becomes equal to a product mass.

Oxygen gas in a gas tank has an inital temperature of 325 K, and a pressure of 5 atm. If the gas is cooled to 280K, what will the new preasure be? (Gay-Lussac's Law)
Answers
Answer:
Final pressure = \(4.31atm\)
Explanation:
According to Gay-Lussac's law the pressure of a given mass of gas varies directly with the absolute temperature of the gas, provided the volume is kept constant.
SEE THE ATTACHMENT BELOW FOR STEP BY STEP EXPLANATION

Given that there are more possible combinations for amino acids than amino acids themselves, what does this imply about the number of codes for each amino acid?
Answers
The fact that there are more possible combinations for amino acids than the number of amino acids themselves implies that each amino acid can be encoded by multiple codons.
A codon is a sequence of three nucleotides in DNA or RNA that corresponds to a specific amino acid.
There are 20 standard amino acids used to build proteins in living organisms. However, there are 64 possible three-letter combinations of nucleotides (4³) that can be used to form codons. This means that on average, there are more than three codons that can encode each amino acid.
To calculate the number of codons per amino acid on average, we divide the total number of codons (64) by the number of amino acids (20). Therefore, the average number of codons per amino acid is 64/20 = 3.2.
This indicates that there is a degeneracy or redundancy in the genetic code, where multiple codons can specify the same amino acid.
For example, the amino acid leucine is encoded by six different codons (UUA, UUG, CUU, CUC, CUA, CUG), while methionine and tryptophan are each encoded by a single codon.
In conclusion, the existence of more possible combinations for amino acids than amino acids themselves means that the genetic code is degenerate, allowing for redundancy and flexibility in protein synthesis.
This redundancy helps to protect against errors in DNA replication and transcription and allows for evolution to occur through the accumulation of genetic variations.
To know more about codons visit:
https://brainly.com/question/26929548
#SPJ11
significant figures to 2.3 x 4.50
Answers
Answer:
10.35= 4 sig figs but change it to 2 since it's 2.3 is the lowest number
10.
hope this helps
have a good day :)
Explanation: