a. How many moles of sodium bicarbonate were present in Beaker "B" in Trial 1 (this was before any acid was added)? Show your work and include units. .500g of sodium bicarbonate were added to 100.0 ml of water b. What is the balanced molecular equation for the neutralization reaction between acetic acid and sodium bicarbonate (these are the reactants)? Include correct formulas, coefficients and physical states.. Hint: One product of this reaction is carbonic acid which is highly unstable and immediately decomposes into carbon dioxide and water. C. Based on your answer to Part b, what is the mole ratio of acetic acid to sodium bicarbonate? d. Based on your answer to Part c, how many moles of acetic acid are required to neutralize all of the sodium bicarbonate? Show your work and include units. Neutralization of Acids and Bases Page 8 of 8 e. Based on your answers to Parts a-d, if a stronger solution of sodium bicarbonate was used in beaker "B," would it require more or less acetic acid to neutralize? Explain why in 2-3 sentences.
Answers
The number of moles of sodium bicarbonate present in Beaker "B" in Trial 1 is 0.00508 moles, The mole ratio of acetic acid to sodium bicarbonate is 2:1.
a. The number of moles of sodium bicarbonate present in Beaker "B" in Trial 1 is 0.00508 moles. This can be calculated using the following equation:
Moles = Mass (g) / Molar Mass (g/mol)
0.00508 moles = 0.500g / 98.0g/mol
b. The balanced molecular equation for the neutralization reaction between acetic acid and sodium bicarbonate is:
2HAc + NaHCO3 → NaAc + H2O + CO2
where HAc is the molecular formula for acetic acid, NaHCO3 is the molecular formula for sodium bicarbonate, NaAc is the molecular formula for sodium acetate, H2O is the molecular formula for water, and CO2 is the molecular formula for carbon dioxide.
c. The mole ratio of acetic acid to sodium bicarbonate is 2:1.
d. To neutralize all of the sodium bicarbonate, 0.00254 moles of acetic acid are required. This can be calculated using the following equation:
Moles acetic acid = Moles sodium bicarbonate * Mole ratio acetic acid:sodium bicarbonate
0.00254 moles = 0.00508 moles * 2:1
e. If a stronger solution of sodium bicarbonate was used in beaker "B," it would require less acetic acid to neutralize. This is because a stronger sodium bicarbonate solution would have a higher concentration of moles of sodium bicarbonate than a weaker solution, so fewer moles of acetic acid would be needed to balance the reaction.
To learn more about moles here:
https://brainly.com/question/29367909#
#SPJ11
Related Questions
What are the correct coefficients when the equation is balanced? _NaF + _Br2 --> _NaBr + _F2
Answers
The balanced equation for the reaction between sodium fluoride and bromine gas is _NaF + _Br2 → _NaBr + _F2 Balancing chemical equations.
The following procedure is commonly used to balance chemical equations:
Step 1: Begin by writing the unbalanced equation.
Count the number of atoms of each type on both the reactant and product sides.
Step 2: Choose an element to balance first.
Usually, the element that appears in only one reactant and one product is selected.
Oxygen is frequently selected because it is present in almost all compounds.
For polyatomic ions, balance the elements contained within the ion as a single unit.
Step 3: Balance the element you chose in step 2 by adding the correct coefficient to the appropriate compound.
Make sure that you do not alter the chemical formula of the compound.
Step 4: Repeat the previous steps until all elements in the equation are balanced.
The correct coefficients when the equation is balanced are:
1 NaF + 1 Br2 → 1 NaBr + 1 F2
To know more about Balancing chemical visit:
https://brainly.com/question/28294176
#SPJ11
what is the mass/volume percent of a solution prepared from 15.0 g nacl in 75.0 g solution? the density of the solution is 1.00 g/ml.
Answers
20% is the mass/volume percent of a solution prepared from 15.0 g NaCl in 75.0 g solution if the density of the solution is 1.00 g/ml.
The volume percentage of a component = (Volume of the component/Total volume of the solution) x 100
Mass by volume percentage: It is the mass of solute dissolved in 100 mL of the solution.
The measure of how densely a material is packed together is called density. As the mass per unit volume, it has that definition. Density Formula: = m/V, where is the density, m is the object's mass, and V is its volume.
mass of solute = 15.0 g of NaCl
mass of solvent = 75.0 g of water
solute + solvent = 15.0+ 75.0 = 90g
(15g/90g) x 100 = 20%
To learn more about volume percentage, please visit -
https://brainly.com/question/29278275
#SPJ4
physical and chemical proportys
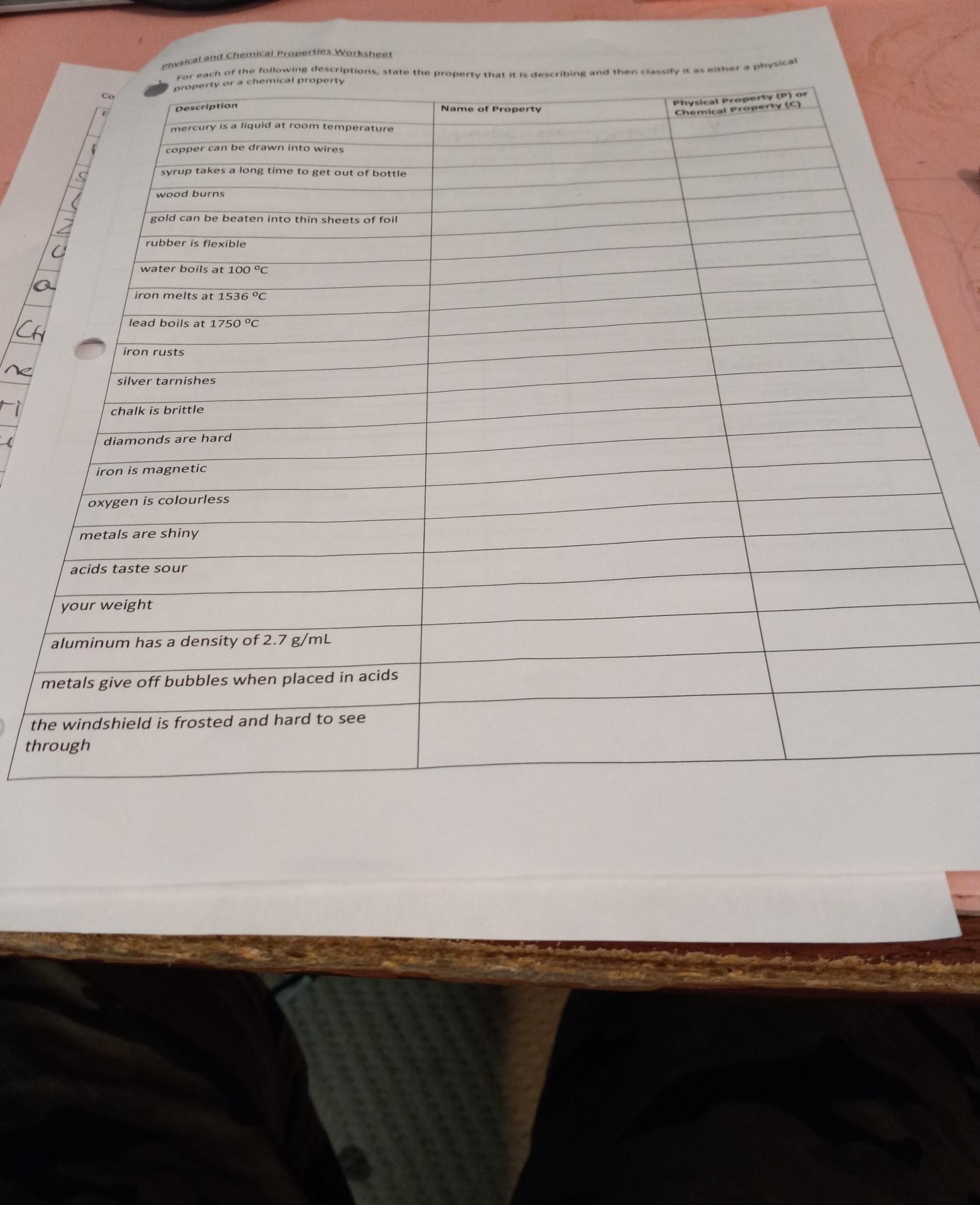
Answers
The physical and the chemical properties of matter are listed below.
What are the examples of physical and chemical properties?While the entire page is not clear, I have an idea of what you are trying to ask and I would show you the physical and the chemical properties of matter.
Physical properties are characteristics of a substance that can be observed or measured without changing the substance's chemical composition. Examples of physical properties include:
Density
Color
Boiling point
Melting point
Hardness
Solubility
Electrical conductivity
Optical properties (such as refractive index)
Chemical properties are characteristics of a substance that describe its ability to undergo chemical reactions and form new substances. Examples of chemical properties include:
Reactivity with other substances
Combustibility
Oxidation state
Acidity or basicity (pH)
Toxicity
Flammability
Learn more about matter:https://brainly.com/question/28487167
#SPJ1
Calculate the maximum wavelength of light capable of dissociating the f–f bond in one molecule of fluorine if the bond energy, or bond dissociation energy, is 157 kj/mol.
Answers
The calculated maximum wavelength is 495 nm
The energy needed to break a bond and create two atomic or molecular fragments, each containing one of the original shared pair of electrons, is known as the bond dissociation energy. The link between silicon and fluorine, which was previously discussed, is discovered to be the strongest chemical bond. The initial silicon-fluorine bond in a silicon tetrafluoride molecule requires 166 kcal/mol of bond dissociation energy to be broken.
ΔH=+240 kJ/mol
E=242×103/6.022×1023
=4.0186×10−19 J
Now I use the Planck Expression:
E=hf=hc/λ
∴λ=hc
Eλ=6.63×10−34×3×108/4.0186×10-19 m
λ=4.949×10−7 m
λ=495 nm.
Learn more about dissociation energy here-
https://brainly.com/question/20475991
#SPJ4
Determine physiological temperature, 98.6 F in degree C
Answers
Answer:
37
Explanation:
( 98.6 - 32 ) × 5(100c) ÷ 9(180f) = 37
the mass of a calibrated flask is 25.0 g when empty, 75.0 g when filled with water, and 88.0 g when filled with glycerin. find the specific gravity of glycerin.
Answers
The specific gravity of the glycerin, given that the mass of a calibrated flask is 25.0 g, is 1.26
How do I determine the specific gravity of the glycerin?First, we shall determine the volume of the caliberated flask by obtaining the volume of the water. This shown below:
Mass of caliberated flask = 25 gMass of caliberated flask + water = 75 gMass of water = 75 - 25 = 50 gDensity of water = 1 g/mLVolume of water =?Volume = mass / density
Volume of water = 50 / 1
Volume of water = 50 mL
Thus, the volume of the caliberated flask is 50 mL
Next, we shall determine the density of the glycerin. This shown below:
Mass of caliberated flask = 25 gMass of caliberated flask + glycerin = 88 gMass of glycerin = 88 - 25 = 63 gVolume of caliberated flask = 50 mLVolume of glycerin = Volume of caliberated flask = 50 mLDensity of glycerin =?Density = mass / volume
Density of glycerin = 63 / 50
Density of glycerin = 1.26 g/mL
Finally, we shall determin the specific gravity of the glycerin. Details below:
Density of water = 1 g/mLDensity of glycerin = 1.26 g/mLSpecific gravity of glycerin =?Specific gravity of glycerin = Density of glycerin / Density of water
Specific gravity of glycerin = 1.26 / 1
Specific gravity of glycerin = 1.26
Learn more about specific gravity:
https://brainly.com/question/28960105
https://brainly.com/question/9895615
#SPJ1
Drag each tile to the correct image.
Match each alkane name with its structure.
octane
decane
propane
butane
heptane
CHE
IGH
CHE
Reset
Next

Answers
Answer:
The first one is Propane
The second one is HEPTANE
The third one is octane
The 4th is butane
the 5th is decane
The structures have been named according to IUPAC as \(\rm C_3H_8\) Propane, \(\rm C_7H_{16}\) Heptane, \(\rm C_8H_{18}\) Octane, \(\rm C_4H_{10}\) Butane, and \(\rm C_{10}H_{22}\) Decane.
The images has been the representation of the ball and stick structure of the compounds. The central balls have been the representation of the carbon atom , with small balls attached to the sticks have been the representation of the hydrogen attached.
The following structures has been given as:
The structure has 3 carbon atoms with the presence of 8 hydrogen. The molecular formula has been \(\rm C_3H_8\). It has been the structure of propane.The structure has 7 carbon and 16 hydrogen. The structure has been the representation of heptane with molecular formula \(\rm C_7H_{16}\).The structure has molecular formula \(\rm C_8H_{18}\) with 8 carbon and 18 hydrogen. It has been named Octane, according to IUPAC.The structure with 4 carbon and 10 hydrogen with molecular formula \(\rm C_4H_{10}\) has been named according to IUPAC as butane.The structure with molecular formula \(\rm C_{10}H_{22}\) has presence of 10 carbon and 22 hydrogen. It has been named as Decane.The structures have been named according to IUPAC as \(\rm C_3H_8\) Propane, \(\rm C_7H_{16}\) Heptane, \(\rm C_8H_{18}\) Octane, \(\rm C_4H_{10}\) Butane, and \(\rm C_{10}H_{22}\) Decane.
For more information about structure of hydrocarbons, refer to the link:
https://brainly.com/question/8049265
Is spoiling milk chemical change?
Answers
No, spoiling milk is not a chemical change. Spoiling milk is a physical change, which is a change in the state or appearance of a substance without changing its chemical composition.
When milk spoils, it is still composed of the same molecules, but its physical properties (such as taste, smell, and texture) have changed. Spoiling milk is caused by the growth of microorganisms, such as bacteria, which break down milk proteins and fats, causing changes in the milk's appearance, taste, and smell. In the case of milk, spoiling is the result of the growth of bacteria, which is a physical change. A physical change is a change in the physical state or appearance of a substance without any change in the chemical composition or identity of the substance.
To learn more about physical change click here https://brainly.com/question/17931044
#SPJ4
1. Examine the two scenarios below.
• Scenario A: 1 mole of glucose in the presence of unlimited oxygen is metabolized through aerobic
respiration
• Scenario B: 1 mole of glucose in the absence of oxygen is metabolized through anaerobic
respiration
Would the number of moles of carbon dioxide produced in Scenario A be greater than, less than or equal to
the number of moles of carbon dioxide produced in Scenario B? Justify your answer.
Upload an image of your response by clicking "Upload files" or by dragging and dropping your file Into the
box. Or use your device's camera to take a photo of your work by clicking the camera Icon.
1
a
T
Upload files
Supported file formats: PDF, JPG, PNG, RTF,TXT, ZIP, Word, Publisher, Open Office, Excel
0/3 File Limit
2. Examine the two scenarios below.

Answers
In the presence of excess oxygen, there is a greater production of CO2 and ATP molecules compared to the absence of oxygen in anaerobic
respiration.
What is glucose?Glucose is the energy molecule that is the end product of the digestion of carbohydrates. During cellular respiration, the glucose molecule is broken down to release carbon dioxide, water and ATP.
In the presence of excess oxygen, there is a greater production of CO2 and ATP molecules compared to the absence of oxygen in anaerobic
respiration.
Learn more about carbon dioxide: https://brainly.com/question/3049557
i.) Draw the lewis structure for [CH2NH2]+ ii.) Draw all the resonance structures iii.) Determine the major contributor
Answers
Resonance happens when there is a single pair or numerous bonds with a positive and negative charge. Only two structures formed in this instance, with the second structure being more stable than the first.
When another object's vibrations match those of an object, resonance happens, amplifying the oscillations of the first object. In physics, resonance is described as follows: a phenomena when a vibrating system or external force causes another system nearby to vibrate more intensely at a particular operating frequency Lone pairs, also known as unshared pairs or non-bonding pairs, are valence electron pairs in chemistry that are not shared with another atom in a covalent connection.
Learn more about Resonance here
https://brainly.com/question/29547999
#SPJ4
![i.) Draw the lewis structure for [CH2NH2]+ ii.) Draw all the resonance structures iii.) Determine the](https://i5t5.c14.e2-1.dev/h-images-qa/answers/attachments/B7FEz4MYRPxVzGhfQ4KYaKnb5Eb0s4K1.png)
draw the lewis structure of so₃ (with minimized formal charges) and then determine the hybridization of the central atom.
Answers
The Lewis structure of SO₃ with minimized formal charges can be drawn as follows:
O
||
O -- S -- O
||
O
To minimize the formal charges, the double bond between sulfur and one of the oxygen atoms is shifted to form a double bond between sulfur and the other oxygen atom, resulting in a structure with three equivalent resonance structures.
To determine the hybridization of the central atom, we can count the number of electron groups (bonded atoms and lone pairs) around the sulfur atom. In SO₃, sulfur is bonded to three oxygen atoms, and there are no lone pairs on the central atom. Therefore, there are a total of 3 electron groups around the sulfur atom.
Learn more about The Lewis structure: https://brainly.com/question/29603042
#SPJ11
When will the simping end

Answers
Answer:
Nvr XD
Explanation:
Answer:
the world may never know
Explanation:
Can someone tell me how to solve this #17 #18 ? Please

Answers
Explanation:
17. They reach to form Fe2O3
18. I think they dont reach
Which animals/insects look different from their young?
Answers
The Principal Investigator (PI) who conducts recombinant or synthetic nucleic acid molecule research is responsible for full compliance with the NIH Guidelines during the conduct of such research. As part of this general responsibility the PI shall:
Answers
As part of this general responsibility, the Principal-Investigator (PI) shall do the following: Notify the NIH Office , Conduct initial and ongoing assessments,Ensure that all laboratory personnel are properly trained, Provide necessary medical attention etc.
The responsibilities of PI(Principal Investigator) who conducts recombinant or synthetic nucleic acid molecule research is responsible for full compliance with the NIH Guidelines during the conduct of such research are as follows:
• Notify the NIH Office of Biotechnology Activities (OBA) immediately upon the identification of any significant problems, violations of the NIH Guidelines, or any significant research-related accidents and illnesses.
• Conduct initial and ongoing assessments of the risks associated with the use of recombinant or synthetic nucleic acid molecules and implement appropriate biological safety practices, containment systems, and emergency procedures.
• Ensure that all laboratory personnel are properly trained in handling recombinant or synthetic nucleic acid molecules.
• Establish appropriate procedures for the proper physical containment of recombinant or synthetic nucleic acid molecules.
• Conduct periodic reviews of the research, especially in cases where significant new information regarding the risk to laboratory personnel, public health, or the environment arises.
• Provide necessary medical attention, including immunizations and medical surveillance, to laboratory personnel working with recombinant or synthetic nucleic acid molecules.
• Establish and follow guidelines for the safe disposal of contaminated materials.
To know more about Principal-Investigator, visit:
brainly.com/question/28498432
#SPJ11
how many grams of hf form from the reaction of 22.2g of nh3 with an excess of fluorine
Answers
When 22.2g of NH₃ reacts with an excess of fluorine, 26.0 g of HF form. The balanced equation for this reaction is: NH₃ + F2 → HF + NHF₂
1. Calculate the molar mass of NH₃ and HF; Molar mass of NH₃ = 14.01 + 1.01 × 3 = 17.04 g/mol Molar mass of HF = 1.01 + 18.99 = 20.00 g/mol
2. Determine the number of moles of NH₃ used. Moles of NH₃ = 22.2 g ÷ 17.04 g/mol = 1.30 mol
3. Find the limiting reactant NH₃ + F₂ → HF + NHF₂
For every mole of NH₃ that reacts with F₂, one mole of HF is produced. Therefore, 1.30 mol of NH₃ will produce 1.30 mol of HF.
4. Calculate the number of moles of HF formed. Number of moles of HF = number of moles of NH₃ used = 1.30 mol5. Calculate the mass of HF formed. Mass of HF = number of moles × molar mass= 1.30 mol × 20.00 g/mol= 26.0 g
Therefore, 22.2g of NH₃ reacts with an excess of fluorine to form 26.0 g of HF.
To know more about fluorine, refer
https://brainly.com/question/15045637
#SPJ11
4. In the following reaction: 2NaN3 decomposes to form 2Na + 3N2. If 500 grams of NaN3 decomposes to form 320 grams of Na, how much Na is produced?
180 grams
320 grams
500 grams
Answers
mass of NaN₃ → moles of NaN₃ → moles of Na → mass of Na
500 g NaN₃ ×
1
mol NaN₃
65.01
g NaN₃
= 7.69 mol NaN₃
7.69 mol NaN₃ ×
2
mol Na
2
mol NaN₃
= 7.69 mol Na
7.69 mol Na ×
22.99
g Na
1
mol Na
= 176.8 g Na
Using Mass of N₂
You must make the following conversions:
mass of N₂ → moles of N₂ → moles of Na → mass of Na
323.20 g Na ×
2
mol N₂
28.01
g N₂
= 11.539 mol N₂
11.539 mol N₂ ×
2
mol Na
3
mol N₂
= 7.692 mol Na
7.692 mol Na ×
22.99
g Na
1
mol Na
= 176.85 g Na
A cylinder of gas has a pressure of 4. 882 atm on one day. The next day, the same cylinder of gas has a pressure of 4. 690 atm, and its temperature is 8. 0 degrees celsius. What was the temperature (in degrees celsius) on the previous day?.
Answers
A cylinder of gas has a pressure of 4. 882 atm on one day, the temperature (in degrees celsius) on the previous day was 20 °C.
A unit called temperature is used to represent hotness or coolness on a variety of arbitrary scales. Additionally, it illustrates the transfer of heat energy organically.
We formula for,
\(\frac{P1}{T1} = \frac{P2}{T2}\)
We have,
Day one's pressure is 4.882 atm.
The following day's pressure is 4.690 atm.
The next day, with a pressure of 4.690 atm, the temperature is 8 °C.
We convert the degree into Kelvin,
8 + 273 = 281 K
Putting the values in the equation
\(\frac{4.882}{T1} = \frac{4.690}{281}\)
We get, T1 = 292.5K
293 K converting into Celsius
293 - 273 = 20 °C
Thus, the temperature on the previous day is 20 °C.
Learn more about Temperature questions:
https://brainly.com/question/15694310
#SPJ4
A student was testing an unknown element to see if it was a metal or non metal. The element was solid, dull in appearance, very brittle , and conducted electricity. What was this unknown element?
Answers
Answer:
The element was one of the alkaline metals, the dull appearance comes from the metal reacting with oxygen and oxidizing its outer surface as alkaline are known for their enormous reaction with oxygen in the air and water.
Another ability of this group of elements is the ability to conduct electricity. And they are also easy to cut with a kitchen knife.
The molar mass of sulfur S is 32.06 g/mol
Calculate the mass in grams of a sample of S containing 2.01x10^24 atoms
Help Please<3
Answers
Since we are starting from the number of atoms of Sulfur, we need to know two sets of formulas:
mass = moles × molar mass moles = atoms ÷ Avogadro's Number
⇒ mass = (atoms ÷ Avogadro's Number) × molar mass
mass = [(2.01 × 10²⁴ atoms) ÷ (6.022 × 10²³ atoms/mole)] × (32.06 g/mol)
= 107.01 g
the mass of a sample of S containing 2.01×10²⁴ atoms is 107.01 g.The molar mass of sulfur S is 32.06 g/mol, then the mass in grams of a sample of S containing 2.01x10^24 atoms is 107.01 g
what is molar mass ?The molar mass can be defined as the weight of one sample mole, by Multiplying the subscript means the number of atoms times that element’s atomic mass and add the masses of all the elements in the molecule to obtain the molecular mass.
Molar mass is expressed in either gram ( g) or kilograms (kg).
Mass = moles × molar mass
moles = atoms ÷ Avogadro's Number
⇒ mass = (atoms ÷ Avogadro's Number) × molar mass
mass = [(2.01 × 10²⁴ atoms) ÷ (6.022 × 10²³ atoms/mole)] × (32.06 g/mol)
= 107.01 g
Learn more about molar mass, here:
https://brainly.com/question/22997914
#SPJ2
Calculate the total number of protons, neutrons, and electrons for Bromine (Br).
Answers
Answer:
protons=35 neutrons=44.9 electrons=35
Explanation:

In a classroom, which comparison would a teacher most likely use for describing a mole?
Answers
Answer:
If you were speaking of the term mole as a skin condition then a teacher might compare it to a beauty spot. In the position your were speaking of it as an animal they might compare it to mouse a rat or a hamster. In the case you were speaking of a mole as a secret agent then a teacher might compare them to a double agent.
mandatory drug testing for high school athletes pros and cons
Answers
Mandatory drug testing for high school athletes is a practice that involves the testing of athletes for illegal substances. This is done to ensure that high school athletes remain drug-free. This practice has its pros and cons. Pros of mandatory drug testing for high school athletes.
Helps to deter drug use: When high school athletes are subjected to mandatory drug testing, it helps to deter drug use. The fear of being caught using drugs can discourage students from using them. This, in turn, promotes a environment in high schools.
Prevents drug use among student-athletes: Mandatory drug testing ensures that student-athletes are drug-free. This is important because drug use can negatively affect the performance of athletes and also affect their health. Cons of mandatory drug testing for high school athletes.
Costly: Mandatory drug testing can be very expensive. This cost is usually borne by the school and can be quite burdensome. This can lead to other important school programs being neglected. Unreliable: The tests used in mandatory drug testing are not always reliable. False positives can occur, leading to innocent students being wrongly punished. This can lead to a decrease in trust between the students and school officials.
In conclusion, mandatory drug testing for high school athletes has its pros and cons. While it helps to deter drug use and prevent drug use among student-athletes, it can also be costly and unreliable.
To know more about illegal substances, visit:
https://brainly.com/question/32373506
#SPJ11
5. A recently named element is Darmstadtium (Ds) which has Z = 110 electrons. Assume that all the electrons can be assigned one by one into the atomic shells with negligible electron-electron interact. With the atom in ground state, what is the spectroscopic notation for the quantum number l for the last electron?
Answers
The spectroscopic notation for the quantum number l for the last electron in Darmstadtium (Ds) is s, since the last electron is in the 7s subshell.
It determined by the total number of electrons in the atom. The quantum number l corresponds to the orbital angular momentum of the electron.
In the case of Darmstadtium (Ds) with Z = 110 electrons, we can determine the spectroscopic notation for the last electron as follows:
First, we need to determine the electron configuration of Darmstadtium. Since Z = 110, the electron configuration can be written as [Rn]5f¹⁴ 6d⁹ 7s¹, where [Rn] represents the electron configuration of the previous noble gas, radon (Rn).
Next, we need to identify the shell and subshell for the last electron. In this case, the last electron is in the 7s subshell, which corresponds to the n = 7 shell.
The spectroscopic notation for the quantum number l is given by the letters s, p, d, f, corresponding to l = 0, 1, 2, 3, respectively. Since the last electron is in the 7s subshell, which has l = 0, the spectroscopic notation for the quantum number l for the last electron in Darmstadtium is s.
To know more about the spectroscopic notation refer here :
https://brainly.com/question/20567097#
#SPJ11
Explain the concept law of diminishing marginal rate of substitution. What is/are the reason/s why the law of diminishing marginal rate of substitution suggest/s that isoquant must be bent toward the origin?
Answers
The law of diminishing marginal rate of substitution indicates that the rate at which one input can be substituted for another decreases as the quantity of one input increases, leading to isoquants being bent toward the origin.
In other words, as the quantity of one good increases, the individual is willing to sacrifice fewer units of the other good to obtain an additional unit of the first good. This reflects a diminishing rate of substitution between the two goods.
The reason why the law of diminishing marginal rate of substitution suggests that isoquants must be bent toward the origin is rooted in the concept of diminishing marginal utility. As more units of a particular input (e.g., labor or capital) are added while holding other inputs constant, the additional output gained from each additional unit of the input will decrease. This diminishing marginal productivity leads to a decreasing MRS.
When isoquants (which represent different combinations of inputs that produce the same level of output) are bent toward the origin, it reflects the fact that as more of one input is used, the amount of the other input that needs to be substituted decreases. This bending signifies the diminishing MRS and captures the idea that a larger quantity of one input can be substituted for a smaller quantity of the other input to maintain the same level of output.
Overall, the law of diminishing marginal rate of substitution indicates that the rate at which one input can be substituted for another decreases as the quantity of one input increases, leading to isoquants being bent toward the origin.
To know more about marginal rate of substitution, click here, https://brainly.com/question/30763866
#SPJ11
What chlorine concentration should be produced when disinfecting a well or well pump?
a.) 25 mg/L
b.) 50 mg/L
c.) 75 mg/L
d.) 100 mg/L
Answers
The recommended chlorine concentration for disinfecting a well or well pump is 50 mg/L.
When disinfecting a well or well pump, a chlorine concentration of 50 mg/L (option b) is typically recommended to ensure effective disinfection and removal of contaminants.
A highly reactive material is chlorine. There is no disinfection at this point when it is introduced to a well; instead, it initially interacts with inorganic substances (hydrogen sulphide, ferrous iron, and manganese). Afterwards, the leftover chlorine reacts with organic matter (algae, phenols, and slime growth) as a result of the reduction of these chemicals. While some unpleasant flavours and aromas might be eradicated, there is only a weak disinfectant activity, and trihalomethanes (carcinogenic, chlorinated organics) might be created.
To know more about chlorine concentration click here:
https://brainly.com/question/10505077
#SPJ11
When 6 moles of Fe react with excess of O2, how many moles of Fe3O4 can be produced?

Answers
Answer: 2 moles Fe3O4
Explanation:
6 moles Fe X (1 mole Fe3O4 / 3 moles Fe) = 2 moles Fe3O4
Multiple Choice. For each statement, circle the correct answer.
9. Most of the energy that drives the water cycle comes from
a. The Sun
b. Earth's cores
c. Earth's oceans d. the equator
10.
is the driving force behind excess runoff after a big precipitation event.
a. precipitation b. steepness of a hill c. gravity
d. solar radiation
Answers
9. A
10. C
The synthesis of nylon requires solutions of 5% hexamethylenediamine and 5% adipoyl chloride. This polymer will form Choose... To remove the nylon, Choose... Choose... in the 5% hexamethylenediamine in the 5% adipoyl chloride in between layers of the solutions
Answers
The synthesis of nylon requires solutions of 5% hexamethylenediamine and 5% adipoyl chloride. This polymer will form between layers of the solutions. To remove the nylon, one can choose to dissolve it in the 5% hexamethylenediamine or in the 5% adipoyl chloride.
Nylon, a synthetic polymer, is produced from the combination of adipoyl chloride and hexamethylenediamine. This process is called the synthesis of nylon. Nylon is a highly flexible material that is resistant to wear and tear, as well as chemical and heat degradation. The synthesis of nylon requires solutions of 5% hexamethylenediamine and 5% adipoyl chloride, respectively, for the two reactants to be mixed together.
The reaction between these two chemicals is exothermic, which means that it releases heat. The heat generated in the reaction drives the reaction forward, resulting in the formation of nylon. The chemical formula for nylon is (-CO-NH-)n, where n is a large number that reflects the degree of polymerization. To remove the nylon, it is soaked in an acid solution. The acid dissolves the nylon, separating it into its constituent components, which can then be purified and reused.
The most commonly used acid for this process is hydrochloric acid. The process of removing nylon from its solvent is called the "acid-catalyzed hydrolysis of nylon." Nylon is used in a variety of applications, including textiles, packaging materials, and electrical components, among others. Its properties make it ideal for use in applications that require durability, strength, and flexibility. Nylon's physical properties, including its resistance to heat and chemical degradation, make it ideal for use in applications such as electrical insulation, packaging materials, and textiles.
Know more about nylon here:
https://brainly.com/question/25835424
#SPJ8
A rod measuring 13.870000 x 3.640000 x 5.980000 cm was plated by means of a current of 84.780000 milliamps for 3.670000 hours. What is the thickness in millimeters of the silver deposit on the rod, given that the density of silver is 10.650000 g / cm3
Answers
Answer:
0.024 mm
Explanation:
The quantity of charge deposited Q = It where I = current = 84.780000 mA = 0.084780000 A and t = time = 3.670000 hours = 3.670000 × 3600 s = 132120000 s.
Also Q = nF where n = number of moles of electrons silver deposited and F = Faraday's constant = 96500 C/mol
So, It = nF
n = It/F = 0.084780000 A × 132120000 s/96500 C/mol = 1120.1134 C/96500 C/mol = 0.012 mol
So, we have 0.012 mol of electrons
Our chemical equation is
Ag⁺ + e⁻ → Ag
Since 1 mol of electrons deposits 1 mol of silver atoms, then, 0.012 mol of electrons deposits 0.012 mol of silver atoms.
Since number of moles of silver atoms, n' = m/M where m = mass of silver atoms deposited and M = molar mass of silver = 107.868 g/mol
So, m = n'M
since n' = 0.012 mol,
m = 0.012 mol × 107.868 g/mol = 1.294 g
Since density of silver ρ = m/V where m = mass of silver deposited = 1.294 g and V = volume of silver deposited
V = m/ρ
Since, ρ = 10.650000 g/cm³
V = 1.294 g/10.650000 g/cm3 =
V = 0.122 cm³
Since the dimensions of the measuring rod are 13.870000 x 3.640000 x 5.980000 cm which represent its length, l, width, w and height, h respectively, the volume of silver deposited V = Ah' where A = area of the rod, lw and h' = thickness of silver deposited
So, V = Ah
V = lwh'
h' = V/lw
= 0.122 cm³/13.870000 cm x 3.640000 cm
= 0.122 cm³/50.4868 cm²
= 0.0024 cm
= 0.024 mm