a structural ____________ is one of several isomers in which the atoms of each molecule are arranged in a different structural order.
Answers
A structural isomer is one of several isomers in which the atoms of each molecule are arranged in a different structural order.
Isomers are molecules that have the same molecular formula but differ in their arrangement of atoms or bonds. Structural isomers, specifically, have the same number and types of atoms, but the way they are bonded together is different, resulting in different physical and chemical properties.
Chemical compounds that have identical chemical formulae but differ in properties and the arrangement of atoms in the molecule are called isomers. Therefore, the compounds that exhibit isomerism are known as isomers. The word “isomer” is derived from the Greek words “isos” and “meros”, which mean “equal parts”.
To know more about isomers refer https://brainly.com/question/2705480
#SPJ11
Related Questions
2. What is her displacement? How do you compare it with distance?
Answers
Answer:
please send the picture otherwise we won't be able to answer
Which will react with oxygen the fastest?a steel beama steel bridge an iron nailiron powder
Answers
Answer
Iron powder
Explanation
The one with the highest surface area will react with oxygen the fastest.
This is because if the surface area of a reactant is increased; more particles are exposed to the other reactant, and there is a greater chance of particles colliding, which leads to more successful collisions per second. Hence the rate of reaction increases.
The one with the highest surface area is iron powder.
Label each process as a physical or chemical change and state how you know.
fogging a mirror with your breath
breaking a bone
mending a broken bone
burning paper
slicing potatoes for fries
mixing sugar with coffee
frying chicken
a nail rusting
paper ripping
wood burning
mixing water and food coloring
food molding (rotting)
writing on paper
dyeing fabric
Answers
Fogging a mirror with your breath is a physical change because it involves a phase change of water vapor condensing and adsorbing to the mirror surface. Phase changes are physical changes.
Breaking a bone is a physical change insofar as we're focusing on the "breaking" part.
Mending a broken bone, however, is a different story. Bones are living things: They consist of tissues that in turn consist of cells. The actual mending process involves some very complicated biochemistry. Suffice it to say that mending a broken bone would be a chemical change.
Burning paper is a chemical change. Burning anything implies combustion, which is a chemical reaction where some fuel is oxidized (usually by oxygen gas, producing carbon dioxide gas and water vapor).
Slicing potatoes for fries is a physical change. You can slice, dice, smash, mash, stretch, bend, compress, or grind a potato: What you will have is still a potato (okay, there are some chemical changes going on as you're rupturing cells in the process, causing them to release their contents which may participate in chemical reactions). But the key, again, is that cutting up some material doesn't fundamentally change the chemical identity of that material.
Mixing sugar with coffee actually involves two physical processes: the mixing and the (presumed) solvation of the solid sugar particles as they dissolve into the coffee. In either case, either mixing or dissolving would be a physical change. The sugar molecules are still in the coffee and are chemically unchanged.
Frying chicken is a chemical change. In fact, frying chicken likely entails several different types of chemical changes. The common thread among them is that frying involves breaking chemical bonds in and on the chicken by the addition of thermal energy, and new chemical bonds end up being formed. That's the hallmark of a chemical change.
A nail rusting is a chemical change. Rusting is an electrochemical process; the familiar corrosion of iron into rust is, at bottom, a chemical reaction where iron reacts with oxygen to form iron oxides (often catalyzed by the presence of water and salts).
A paper ripping is analogous to slicing potatoes: it's a physical change. If you ripped one sheet of paper into two halves, each half would retain all the chemical properties of the original sheet.
Likewise, wood burning is analogous to burning paper, and as such is a chemical change. Again, combustion is a chemical process.
As we said earlier with sugar in coffee, mixing in itself is a physical process. While it may seem like you've permanently changed the nature of the water by dyeing it with food coloring, the molecules comprising the food coloring are simply dispersed within the vast sea of water molecules. There are no intramolecular bonds that are broken or formed; the chemical identities of all the substances here are preserved. So, this is a physical change.
Food molding (rotting) is a chemical change. Rotting is biochemical decomposition: the chemical bonds that make up the food are broken down by enzymes released by the mold.
Writing on paper, whether it be with a pen, pencil, crayon, or marker, is a physical change. The molecules from the writing instrument are physically stuck to the paper. But unless you're writing on paper by, say, burning letters onto it, there are no chemical changes occurring when the writing instrument meets the paper.
As with writing on paper, dyeing fabric can be a physical change. The dye consists of molecules that interact with light in a way that we perceive a certain color. When dyeing fabric, these molecules are transferred and fixed into the fabric by adsorption, absorption, and other intermolecular phenomena. But the molecules of the dye (and the molecules in the fabric) don't experience any breaking and forming of bonds. All of the substances involved retain their chemical identities.
However, it's possible that, depending on the dye, there may be chemical changes involved. Some dyes, appropriate named "reactive dyes," undergo chemical reactions with their substrate (which, in this case, would be the fabric), or dyes may be used that undergo chemical reactions with one another, both of which would constitute chemical changes. And it can depend on what you mean by "dyeing": Bleaching a colored shirt can technically be conceived of as "dyeing" the shirt white, and this process involves cleavage of bonds within the color-producing molecules in the fabric by reacting with the molecules in the bleach.
So, for dyeing fabric, it can be a physical or chemical change depending on the dye.
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
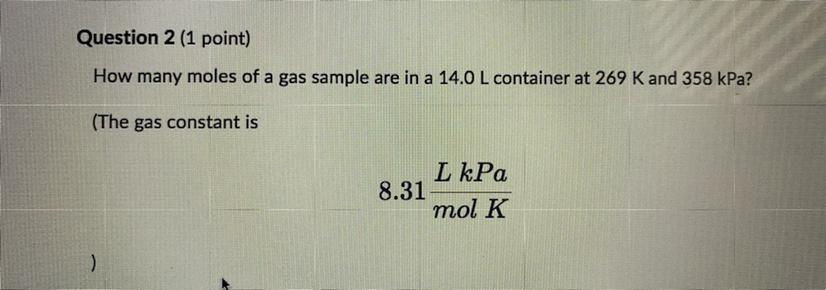
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
Acme Chemical Consultants, a general partnership, discharges from its facility a pollutant prohibited by the Environmental Protection Agency (EPA). In this case, _____.
Multiple Choice
only the partners who ordered the discharge are personally liable
all partners will be imprisoned, as the violation of an EPA standard is a crime per se
the firm is liable for the resulting fines
the general partners are personally liable unless the firm has inadequate assets
Answers
As a general partnership, Acme Chemical Consultants is considered a legal entity separate from its individual partners. general partnership does not provide limited liability protection to its partners.
The partners of Acme Chemical Consultants can be held personally liable for the actions and obligations of the partnership. When the partnership discharges a pollutant prohibited by the EPA, the firm, as a legal entity, will be held responsible for the violation. The EPA has the authority to impose fines and penalties on Acme Chemical Consultants for its actions. The fines levied by the EPA would typically be imposed on the partnership itself, rather than the individual partners.
It is important to note that while the firm is primarily liable for the resulting fine, in certain circumstances, the partners of Acme Chemical Consultants may still be held personally liable if the firm does not have adequate assets to cover the fines. Personal liability of the partners would generally come into play if the partnership is unable to satisfy the imposed fines and the partners are deemed responsible for paying the remaining amount from their personal assets.
Learn more about Acme Chemical here
https://brainly.com/question/16703672
#SPJ11
For strong electrolytes, i = number of per mole of solute dissolved. CaCl dissolves yielding three ions, one Ca ion and two Clions, thus i = (NH. ),P dissolves yielding four ions, three NH' ions and one Pion, thus i = "Colligative Properties Study Guide" by Montgomery College is licensed under CC BY 4. 0
Answers
The statement you provided refers to the determination of the van't Hoff factor (i) for strong electrolytes. The van't Hoff factor represents the number of ions produced per mole of solute dissolved in a solution.
For example, when calcium chloride (CaCl2) dissolves, it dissociates into three ions: one Ca2+ ion and two Cl- ions. Therefore, the van't Hoff factor (i) for CaCl2 is 3 because it produces three ions per mole of solute dissolved.
Similarly, when ammonium phosphate (NH4)3PO4 dissolves, it dissociates into four ions: three NH4+ ions and one PO43- ion. Thus, the van't Hoff factor (i) for (NH4)3PO4 is 4 because it yields four ions per mole of solute dissolved.
The van't Hoff factor is essential in various calculations related to colligative properties, such as boiling point elevation and freezing point depression, where it is used to account for the number of particles in solution.
learn more about electrolytes here
https://brainly.com/question/32477009
#SPJ11
Please help there is a picture below.

Answers
Answer:
A is its answer
Explanation:
Fan uses electrical energy and makes it to mechanical energy by rotating
Same is with the drill
Trampoline does not use electrical energy
Lamp uses electrical energy but it converts it into light energy and heat energy
So A is the answer
Which of the following statements best describes a food web?
A.
Many individuals of the same species that live in the same spaces and that share resources.
B.
A system that is made up of a community of organisms and their environment.
ОООО
C.
A black bear eats fruit and then spreads the fruit's seeds through its excretions
O D.
All life is connected by the transfer of energy and matter among organisms and their environment
Answers
Answer:
Its either B or D. I personally would go with B
Explanation:
A food web describes a system that is made up of a community of organisms and their environment. Therefore, option B is correct.
What is food web ?A food web is a diagram that shows what is eaten by what in an ecological community and how food chains naturally connect to one another. Consumer-resource system is another term for the food web.
A food web is a comprehensive account of the species that make up a community and their interactions with one another. It demonstrates how energy is moved along food chains that are connected to other food chains.
The study of top-down or bottom-up control of community structure can be done using food webs. The energy exchange between primary producers and primary consumers (herbivores) and between primary consumers and secondary consumers is shown through food webs (carnivores).
Thus, option B is correct.
To learn more about food web, follow the link;
https://brainly.com/question/2233704
#SPJ2
Which phrase describes a polymer?
Answers
Answer:
‘many parts’
Explanation:
I remember learning this in last year AP Chemistry
A 684.6 mL sample of carbon dioxide was heated to 387 K. If the volume of the carbon dioxide sample at 387 K is 933.9 mL, what was its temperature at 684.6 mL
Answers
According to Charle's law equation, the volume of a sample of carbon dioxide will be 684.6 mL and the temperature will be 283.60 K.
Charle's law equationAccording to Charles' Law, the volume of a gas container with a certain amount of gas within is precisely proportional to the temperature when the pressure is constant.
Calculation:\(V_{1}\) = 684.6 mL
\(V_{2}\) = 933.9 mL
\(T_{1}\) = ?
\(T_{2}\) = 387 K
Using Charle's law equation
\(\frac{684.6}{T_{1} } = \frac{933.9}{387}\)
\(T_{1} = \frac{2,64,862.8}{933.9}\)
\(T_{1} = 283.60 K\)
According to Charle's law equation, the temperature at 684.6 mL volume was 283.60 K.
For more information about Charle's law equation here
https://brainly.com/question/16927784
#SPJ4
Which salt is the most common found in ocean water?
magnesium
calcium carbonate
potassium
sodium chloride
Answers
Answer:
Sodium chloride
Explanation:
There are several salts in seawater, but the most abundant is ordinary table salt or sodium chloride (NaCl). Sodium chloride, like other salts, dissolves in water into its ions, so this is really a question about which ions are present in the greatest concentration. Sodium chloride dissociates into Na+ and Cl- ions.
I need some help with #2 pls

Answers
The gram-formula mass of the product in the given reaction is 201.8g.
How to calculate gram-formula?Gram Formula mass is the atomic mass of one mole of an element or a molecular compound, or an ionic compound.
To calculate the gram formula mass of a compound, the following applies;
count the number of atoms/ions of each element that is present in one formula unit. Multiply the atomic mass of each element by the total number of atoms/ions of that element present in the formulaAdd all of the masses to obtain the gram formula mass for the compoundAccording to this question, propene reacts with bromine to produce bromo propane with the molecular formula; C₃H₆Br₂.
Gram formula mass = 12(3) + 1(6) + 79.9(2) = 201.8g
Learn more about gram formula mass at: https://brainly.com/question/492953
#SPJ1
NO2 and CO2 have a similar molecular weight. Which gas would you predict to deviate from an ideal
gas? Justify your selection.
Answers
Answer:
NO2
Explanation:
Gases are known to deviate from ideal gas behavior as a result of intermolecular interaction between gas particles. The greater the magnitude of such intermolecular interaction, the greater the degree of deviation of the gas from the ideal gas law.
Now given that CO2 has dipoles that cancel out, the molecule is not really polar because it has no dipole moment. This is unlike NO2 that has a dipole moment and greater intermolecular interaction.
For the reason stated above, NO2 deviates more from ideal gas behaviour compared to CO2.
CO2 has dipoles that cancel out so the molecule is not really polar because it has no dipole moment. NO2 has a dipole moment and greater intermolecular interaction. NO2 deviates more from ideal gas behavior compared to CO2.
What is the difference between a group and a row on the periodic table?
Answers
Answer: A row is horizontal and a group is vertical on the periodic table.
Which situation is does NOT occur during a chemical reaction?
bonds are formed
bonds are broken
mass is conserved
atoms are destroyed
Answers
Steric strain in a large molecule is often reduced by changes in torsion angles. However, in smaller sets of fused benzene rings, like phenanthrene and 3,4-benzophenanthrene (shown below), the first geometric parameters to vary from reference values are found to be the central bond lengths and bond angles. Why do they expand to relieve steric strain before the molecule undergoes torsion to a non-planar structure?
Answers
The molecule expand to relieve steric strain before undergoing torsion to a non-planar structure in order to have several resonating structures.
What is a molecule?It should be noted that a molecule simply mean the group of atoms that are bonded together that represents the smallest fundamental unit if the chemical compound.
It should be noted that structures containing fused benzene rings have extensive conjugation. Due to this, they can undergo resonance.
This is vital in decreasing the overall energy of the molecule and helps increase its stability. When there's a change in torsion angle to relieve the steric strain, the molecule won't be planar, hence, the energy will increase and the stability will reduce.
Learn more about molecules on:
brainly.com/question/26044300
#SPJ1
need asap!
The boiling point of hydrogen sulfide is low. Explain why.
Answers
Sulfur is not nearly as electronegative as oxygen so that hydrogen sulfide is not nearly as polar as water. Because of this, comparatively weak intermolecular forces exist for H2S and the melting and boiling points are much lower than they are in water.
The boiling point of hydrogen sulfide is low because the oxygen atom was stronger electronegative than that of the Sulphur atom, water would be more polar than hydrogen sulphide.
What is polar molecule?If a molecule does have more positive charges on one end than negative charges on the other, an electrical pole has been created. This is how polar molecules are typically formed.
What is boiling point?
The temperature where a liquid's vapor pressure reaches the pressure around it as well as the liquid transforms into a vapor is known as the boiling point of a substance.
The boiling point of hydrogen sulfide is low because the oxygen atom was stronger electronegative than that of the Sulphur atom, water would be more polar than hydrogen sulphide. Additionally, hydrogen sulphide has a lesser melting point as well as boiling point than water due to poor intermolecular forces (dipole forces, Vander Waal's forces).
To know more about polar molecule and boiling point
https://brainly.com/question/3184550.
#SPJ3
The energy on the earth
Answers
Answer:
The earth-atmosphere energy balance is the balance between incoming energy from the Sun and outgoing energy from the Earth. When it reaches the Earth, some is reflected back to space by clouds, some are absorbed by the atmosphere, and some is absorbed in the Earth's surface. ...
Explanation:
Concentration (Volume of solute total volume)(100%) 25 mL of ethanol is added to enough water to make 100.0 mL of solution. Find the percent by volume of ethanol. 2. 50 mL of ethanol is added to 50 mL of water. What is the percent by volume of ethanol?
Answers
The percent by volume of ethanol 2.50 mL of ethanol is added to 50 mL of water is 25%. The percent by volume of ethanol is 50%.
The volume of the solute divided by the total volume of the solution, multiplied by 100%, is a method to express the concentration of a chemical solution as a percentage by volume. Volume percent (vol%) and percent v/v are other names for it. Percent by volume is a unitless number because the volume units cancel out.
To find the percent by volume of ethanol when 25 mL of ethanol is added to enough water to make 100.0 mL of solution, we need to calculate the total volume of the solution first.
Total volume = 25 mL (ethanol) + (100 mL - 25 mL) = 100 mL
Now we can calculate the percent by volume of ethanol:
% by volume of ethanol = (25 mL / 100 mL) x 100% = 25%
Therefore, the percent by volume of ethanol in the solution is 25%.
To find the percent by volume of ethanol when 50 mL of ethanol is added to 50 mL of water, we can again calculate the total volume of the solution first.
Total volume = 50 mL (ethanol) + 50 mL (water) = 100 mL
Now we can calculate the percent by volume of ethanol:
% by volume of ethanol = (50 mL / 100 mL) x 100% = 50%
Therefore, the percent by volume of ethanol in the solution is 50%.
Learn more about percent by volume at https://brainly.com/question/14589084
#SPJ11
What might happen to the cryosphere if the insulating effect of the atmosphere increased?
Answers
Answer:
Acting like a highly reflective blanket, the cryosphere protects Earth from getting too warm. Snow and ice reflect more sunlight than open water or bare ground. Changes in snow and ice cover affect air temperatures, sea levels, ocean currents, and storm patterns all over the world.
Explanation: Hope this answers your question
how many valence electrons does tungsten have
Answers
Answer:74
Explanation:
Answer:
I believe Tungsten has 2 valence electrons.
Explanation:
Hope this helps!
A gas sample contained in a cylinder equipped with a moveable piston occupied 300.0 mL at a pressure of 2.00 atm. What would be the final pressure if the volume were increased to 500.0 mL at constant temperature?
Answers
Answer:
Explanation
hope this helps!

The melting point X of a certain specimen be assumed to be a continuous random variable which is uniformly distributed over the interval [110, 120]. Find density function of X, mean of X, variance of X and P (112 x < 115.).
Answers
Density function: f(x) = 1/10, for 110 ≤ x ≤ 120, and f(x) = 0 otherwise.
Mean: μ = 115.
Variance: σ^2 = 25/3.
Probability: P(112 < X < 115) = 0.3.
Given that the melting point X is uniformly distributed over the interval [110, 120], we can find the density function, mean, variance, and probability as follows:
1. Density function:
Since X is uniformly distributed, the density function f(x) is constant within the interval [110, 120] and zero outside that interval. To find the density function, we need to determine the height of the constant density.
The total length of the interval is 120 - 110 = 10.
Since the density function is constant, the area under the density function curve must be equal to 1.
Therefore, the height of the constant density is 1 divided by the length of the interval: f(x) = 1/10, for 110 ≤ x ≤ 120, and f(x) = 0 otherwise.
2. Mean:
The mean (μ) of a uniform distribution is the average of the endpoints of the interval. In this case, the mean is (110 + 120) / 2 = 115.
3. Variance:
The variance (σ^2) of a uniform distribution is calculated using the formula: σ^2 = (b - a)^2 / 12, where a and b are the endpoints of the interval. In this case, the variance is (120 - 110)^2 / 12 = 10^2 / 12 = 100/12 = 25/3.
4. Probability:
To find P(112 < X < 115), we need to calculate the area under the density function curve between the points 112 and 115.
Since the density function is constant within the interval [110, 120], the probability is equal to the ratio of the length of the interval [112, 115] to the length of the entire interval [110, 120].
The length of the interval [112, 115] is 115 - 112 = 3.
The length of the entire interval [110, 120] is 120 - 110 = 10.
Therefore, P(112 < X < 115) = (3 / 10) = 0.3.
To know more about Density
https://brainly.com/question/29775886
#SPJ11
how many state of matter do we have
Answers
Answer:
3 we have three states of matter
Answer:
We have THREE (3) states of matter namely;
*Solid state
*Liquid state
*Gaseous state
Helllpppp! this is a study island and im stuck :(

Answers
It is clear from the tabular data that, the monkeys in troop 2 were better to avoid predators and more of them were able to reproduce. Hence, option D is correct.
What is natural survival ?In the biosphere, not all living things are fit to survive for a longer life time. Most of them are pray of other higher level animals. Some organisms adopt some strategies to hide from their predators and they can survive more.
It is clear from the table that, the monkeys residing in the ground are more prone to the attacks by their predators. Whereas monkeys living in trees can survive more.
In each year the survival rate is increasing for both troop. However the more number of monkeys which can sustain their population is in troop 2. Therefore, option D is correct.
Find more on natural survival :
https://brainly.com/question/26098341
#SPJ1
What is the empirical formula of a compound that has 40% carbon, 6.7% hydrogen,
and 53.3% oxygen?
Which compound?
Answers
Answer:
Formaldehyde(CH\(_{2}\)O) or methanal is your answer.
En una molécula de cualquier sustancia existe es : *
a) la unión de iones
b) la unión de átomos
c) la unión de electrones
d) la unión de protones
AYUDAAAAAAAAAA
Answers
Las partículas subatómicas se descubrieron durante el siglo XIX. Para nuestros propósitos, nos concentraremos solo en tres de ellos, resumidos en la Tabla 1. El protón está ubicado en el centro (o núcleo) de un átomo, cada átomo tiene al menos un protón. Los protones tienen una carga de +1 y una masa de aproximadamente 1 unidad de masa atómica (uma). Los elementos se diferencian entre sí en el número de protones que tienen, p. Ej. El hidrógeno tiene 1 protón; El helio tiene 2.
El neutrón también se encuentra en el núcleo atómico (excepto en el hidrógeno). El neutrón no tiene carga y tiene una masa de algo más de 1 amu. Algunos científicos proponen que el neutrón está formado por un protón y una partícula similar a un electrón.
El electrón es una partícula muy pequeña ubicada fuera del núcleo. Debido a que se mueven a velocidades cercanas a la velocidad de la luz, es difícil precisar la ubicación precisa de los electrones. Los electrones ocupan orbitales o áreas donde tienen una alta probabilidad estadística de ocurrir. La carga de un electrón es -1. Su masa es insignificante (se necesitan aproximadamente 1800 electrones para igualar la masa de un protón).
Tabla 1. Partículas subatómicas de uso en biología.
Por cierto, tuve que traducir lo que dijiste, así que espero que esto sea bueno, no sé si es la respuesta.
¡buena suerte!
The density of pure solid copper is
2.54 g/mL. What is the mass of 250 milliliters of the substance
Answers
Answer:
The answer is 508 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question we have
mass = 2.54 × 200
We have the final answer as
508 gHope this helps you
Kindly Help Please!
Can you write the symbol equations for each of the reactions below:
and Can you balance them?
1) Ethane + Oxygen = Carbon Dioxide + Water
2) Butane + Oxygen = Carbon Dioxide + Water
3) Octane + Oxygen = Carbon Dioxide + water
4) Decane + Oxygen = Carbon Dioxide + Water
thank you!
Answers
Answer: 1) \(2C_2H_6+7O_2\rightarrow 4CO_2+6H_2O\)
2) \(2C_4H_{10}+13O_2\rightarrow 8CO_2+10H_2O\)
3) \(2C_8H_{18}+25O_2\rightarrow 16CO_2+18H_2O\)
4)\(2C_{10}H_{22}+31O_2\rightarrow 20CO_2+22H_2O\)
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side.
The balanced chemical reactions will be:
1) Ethane + Oxygen = Carbon Dioxide + Water : \(2C_2H_6+7O_2\rightarrow 4CO_2+6H_2O\)
2) Butane + Oxygen = Carbon Dioxide + Water : \(2C_4H_{10}+13O_2\rightarrow 8CO_2+10H_2O\)
3) Octane + Oxygen = Carbon Dioxide + water: \(2C_8H_{18}+25O_2\rightarrow 16CO_2+18H_2O\)
4) Decane + Oxygen = Carbon Dioxide + Water : \(2C_{10}H_{22}+31O_2\rightarrow 20CO_2+22H_2O\)
The Difference Between a Hot Cup of Water and a Cold One in Terms of Thermal Energy
Answers
Answer:
The difference between a hot cup of water and a cold one in terms of thermal energy can be described as the amount of heat energy that is present in each cup. The hot cup of water contains more thermal energy than the cold one due to its higher temperature. This means that the hot cup has more energy to transfer to its surroundings, and will cool down faster than the cold cup. The thermal energy in a cup of water is related to the temperature, with hotter water having a higher thermal energy than colder water.
Explanation: