Answers
Adding enough heat to a system can change the substance in a system
Related Questions
Earth systems can interact quickly or over long periods of time. Give an example of how Earth can change quickly and an example of how it changes slowly. Describe how Earth's systems interact in these changes.
Answers
Answer:
Earth is made of several subsystems or "spheres" that interact to form a complex and continuously changing whole called the Earth system. to thousands of kilometers, and on time scales that range from milliseconds to billions of years of the Earth; earthquake; Examples of long term - making coal; plate tectonics
Explanation:
A man hits a golf ball (0.2kg) which accelerates at a rate of 20 m/s². What amount of
tric force acted on the ball? Complete the table below to show all of your work.
then there’s a triangle with f at the top m in the left and a in the right
Answers
Answer:
F = ma, where F is the force acting on the body, m is the mass of the body, and a is the acceleration of the body. Therefore, the force acting on the ball is 4Newtons.
Explanation:
Use the data in the table below to answer the following questions. The true value for this data set is 5.68g.

Answers
1) The student which is neither precise nor accurate is student B
2) The student which is most accurate on any single trial is student A
3) The student that is both precise and accurate is student C
4) The student which is precise but not accurate is student D
What is precision and accuracy?The term precision has to do with the fact that the values of the measurement are close together. Precise values do not scatter a lot. They vary within a narrow range.
An accurate measurement is one that is close to the true value. This means that it does not differ so much from the true value of the measurement.
Hence;
1) The student which is neither precise nor accurate is student B
2) The student which is most accurate on any single trial is student A
3) The student that is both precise and accurate is student C
4) The student which is precise but not accurate is student D
Learn more about precision and accuracy:https://brainly.com/question/15276983
#SPJ1
The fuel used in many disposable lighters is liquid butane, C4H10 . Butane has a molecular weight of 58.1 grams in one mole. How many carbon atoms are in 1.00 g of butane?
Answers
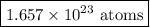
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
An early arrangement of the then known elements was proposed by a British scientist John Newlands, which he called the Law of Octaves. Like other scientists at the time, Newlands arranged the elements in order of increasing atomic mass and noted that every eighth element had similar physical/chemical properties. In the modern Periodic Table, which of the following represents the last pair of elements for which Newlands' Law of Octaves would hold true?
Answers
When of alanine are dissolved in of a certain mystery liquid , the freezing point of the solution is less than the freezing point of pure . Calculate the mass of potassium bromide that must be dissolved in the same mass of to produce the same depression in freezing point. The van't Hoff factor for potassium bromide in .
Answers
The question is incomplete, the complete question is:
When 177. g of alanine \((C_3H_7NO_2)\) are dissolved in 800.0 g of a certain mystery liquid X, the freezing point of the solution is \(5.9^oC\) lower than the freezing point of pure X. On the other hand, when 177.0 g of potassium bromide are dissolved in the same mass of X, the freezing point of the solution is \(7.2^oC\) lower than the freezing point of pure X. Calculate the van't Hoff factor for potassium bromide in X.
Answer: The van't Hoff factor for potassium bromide in X is 1.63
Explanation:
Depression in the freezing point is defined as the difference between the freezing point of the pure solvent and the freezing point of the solution.
The expression for the calculation of depression in freezing point is:
\(\Delta T_f=i\times K_f\times m\)
OR
\(\Delta T_f=i\times K_f\times \frac{m_{solute}\times 1000}{M_{solute}\times w_{solvent}\text{(in g)}}\) ......(1)
When alanine is dissolved in mystery liquid X:\(\Delta T_f=5.9^oC\)
i = Vant Hoff factor = 1 (for non-electrolytes)
\(K_f\) = freezing point depression constant
\(m_{solute}\) = Given mass of solute (alanine) = 177. g
\(M_{solute}\) = Molar mass of solute (alanine) = 89 g/mol
\(w_{solvent}\) = Mass of solvent = 800.0 g
Putting values in equation 1, we get:
\(5.9=1\times K_f\times \frac{177\times 1000}{89\times 800}\\\\K_f=\frac{5.9\times 89\times 800}{1\times 177\times 1000}\\\\K_f=2.37^oC/m\)
When KBr is dissolved in mystery liquid X:\(\Delta T_f=7.2^oC\)
i = Vant Hoff factor = ?
\(K_f\) = freezing point depression constant = \(2.37^oC/m\)
\(m_{solute}\) = Given mass of solute (KBr) = 177. g
\(M_{solute}\) = Molar mass of solute (KBr) = 119 g/mol
\(w_{solvent}\) = Mass of solvent = 800.0 g
Putting values in equation 1, we get:
\(7.2=i\times 2.37\times \frac{177\times 1000}{119\times 800}\\\\i=\frac{7.2\times 119\times 800}{2.37\times 177\times 1000}\\\\i=1.63\)
Hence, the van't Hoff factor for potassium bromide in X is 1.63
a typical baseball has a mass of 350g and takes up a volume of .780L. what is the density of a baseball in g/l and g/ml?
Answers
Answer:
449 g/L
0.449 g/mL
Explanation:
Step 1: Given data
Mass of a typical baseball (m): 350 gVolume of a typical baseball (V): 0.780 LStep 2: Calculate the density (ρ) of the baseball in g/L
The density is equal to the quotient between the mass and the volume.
ρ = m / V
ρ = 350 g / 0.780 L
ρ =449 g/L
Step 3: Convert ρ from "g/L" to "g/mL"
We will use the conversion factor 1 L = 1000 mL.
449 g/L × (1 L/1000 mL) = 0.449 g/mL
consider the fictional case of the incredible shrinking man. if he shrinks proportionately to 1/10 his original height, his weight will be multiplied by question 11 options: 0.1 0.001 0.0001 0.01
Answers
Man weight will be multiplied by 0.001. S o option (b) is correct.
Density is defined as depending on the units of mass and volume used for the calculation, different units are used. The units for density would be kg/cm3 if the mass was measured in kg and the volume in cm3.
Density = Mass divided by volume.
Given a man shrinks proportionality to 1/10 of the original height
Now as it is the proportional overall area will also be decreased by (1/10)^2 = 1/ 100 times
Therefore overall decrease in volume = 1/10 x 1/100
= 1 / 1000 times(v/1000)
Mass/ volume = Q (density)
Here Q remains constant
Hence M initial = Q x V
M final = QV/1000
Initial weight = QV x g
= QVg
Final Weight = (QV/1000) x g
Final /Initial = QVg/1000QVg
= 1/1000
= 0.001
Therefore his weight will be multiplied by 0.001
To learn more about density visit
https://brainly.com/question/29775886
#SPJ4
Whats the Pseudo second order.?
And how we concired the PSO
Answers
A reaction that looks to follow second-order kinetics but is not actually a second-order reaction is described by the pseudo-second order (PSO) model.
A chemical reaction that looks to follow second-order kinetics but is not actually a second-order reaction is referred to as pseudo second order (PSO) in a kinetic model.
It is frequently seen in reactions where one reactant's concentration is substantially higher than that of the other reactant, resulting in an abundance of the abundant reactant that remains essentially constant throughout the reaction.
The rate equation in PSO kinetics has the following structure:
1/t = k * [A] * [B]
where [A] and [B] stand for the reactant concentrations, k for the rate constant, and t for the passage of time. According to the equation, the rate of the reaction is inversely proportional to the product of the reactant concentrations.
The consumption of the limiting reactant, which determines the total reaction rate, is what causes the apparent second-order behaviour. The rate of the reaction falls together with the concentration of the limiting reactant with time.
Adsorption reactions, surface reactions, or situations where one reactant is present in excess of the other are frequently described by the PSO model. The PSO model is an approximation and does not imply a real second-order reaction mechanism, it is vital to remember this.
For more such question on reaction. visit :
https://brainly.com/question/11231920
#SPJ8
A balanced chemical equation has equal numbers of atoms of each type on both sides of the equation. This illustrates the principle of
Answers
Answer:
conservation of mass
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
If you begin with 5.000 grams of KClO3(s) how many moles and KClO3(s)
will be used, and b) how many grams, moles, and molecules of the product species will
be formed?

Answers
Based on the given data, the amount of products from 5.00 g of KClO₃ is:
0.04 moles of KCl2.98 g KCl2.41 * 10²² molecules of KCl 0.06 moles of O₂.1.92 g of O₂3.61 * 10²² molecules of O₂What amount of products is formed from the decomposition of KClO₃?The decomposition of KClO₃ is given by the equation below:
2 KClO₃ -----> 2 KCl + 3 O₂
2 moles of KClO₃ produces 2 moles of KCl and 3 moles of O₂
Molar mass of KClO₃ is 122.5 g/mol
molar mass of KCl = 74.5 g
molar mass of O₂ = 32 g
Moles of KClO₃ in 5.00 g = 5.00/122.5
moles of KClO₃ = 0.04 molesFor KCl
a. moles: 0.04 moles of KClO₃ will produce 0.04 moles of KCl
b. mass of KCl = 0.04 * 74.5 = 2.98 g KCl
c. number of molecules of KCl = 0.04 * 6.02 * 10²³ = 2.41 * 10²² molecules of KCl
For O₂:
a. moles of O₂; 0.04 moles of KClO₃ will produce = 0.06 moles of O₂.
b. mass of O₂ = 0.06 * 32 g = 1.92 g of O₂
c. number of molecules: 0.06 * 6.02 * 10²³ = 3.61 * 10²² molecules of O₂
Therefore, the amount of products from 5.00 g of KClO₃ is:
0.04 moles of KCl2.98 g KCl2.41 * 10²² molecules of KCl 0.06 moles of O₂.1.92 g of O₂3.61 * 10²² molecules of O₂Learn more about moles and molecules at: https://brainly.com/question/26135244
what is the strength of alloys and what is the solubility of alloys please answer this simple question within 12 hours will mark brainliest for sure
Answers
Answer:Features very high tensile strength and toughness. Titanium alloys are light in weight, have superior corrosion resistance properties and can withstand extreme temperatures. ... Titanium alloys are heat-treated to increase their strength in terms of fracture toughness, fatigue strength and high temperature strength.35 grams is the sollubility.
Explanation:
0.175 moles of aluminum nitrate reacted with a sufficient amount of copper sulfate to produce how many moles of copper nitrate
Answers
Copper metal has a mole weight of 0.45 moles. the complementary interaction of copper and aluminum. 0.45 moles of copper nitrate.
Sodium is created when sodium chloride and silver nitrate combine. Next, we must determine how many moles of each reactant there are. This molarity concentration unit connects moles of solute to volume of solution. Chemical processes are expressed in terms of moles of reactants and products. Aluminum oxide is created when aluminum and oxygen react. The lowest set of whole number coefficients in a balanced equation.
to know more about copper nitrate please visit.
https://brainly.com/question/12768349
#SPJ4
Which two statements best describe the methods in the table?
UDP will increase the amount of fertilizer used and help save money on labor.
Precision management allows farmers to save money in the beginning but spend more in the future.
Compared to precision management, UDP will most likely reduce the amount of nitrogen pollution from runoff.
Precision management can help farmers use less fertilizer by targeting specific areas in the farmer's fields.
Answers
By focusing on certain fields, precision management can assist farmers in using less fertiliser. Option D is the right choice as a result.
In 1994, Precision Management was founded with the express purpose of offering customers comprehensive, effective, and personalised property management services while concentrating on and achieving their goals and objectives. Precision has developed friendships as well as commercial ties during the past 25 years. Our clients value Precision's distinctive, hands-on management approach and the company's overall commitment to their assets. By focusing on certain fields, precision management can assist farmers in using less fertiliser.
Therefore, the correct option is option D.
To know more about Precision management, here:
https://brainly.com/question/31721041
#SPJ1
Suppose a student repeats Experiment 1 using strontium instead of magnesium. The student adds 4.93 g of strontium to a crucible, heats the crucible and its contents for several minutes over a Bunsen burner, and records the final mass of the crucible and its contents.
Write the balanced chemical equation for this reaction. Include physical states.
balanced equation:
What mass of product is expected to form in this reaction? Assume all of the strontium reacts.
mass of product:
Answers
The balanced chemical equation for the reaction between strontium and oxygen can be written as follows: 2 Sr (s) + \(O_2\)(g) → 2 SrO (s).
In this equation, solid strontium (Sr) reacts with gaseous oxygen (\(O_2\)) to produce solid strontium oxide (SrO).
To determine the mass of product expected to form in this reaction, we need to consider the molar ratio between strontium and strontium oxide. From the balanced equation, we can see that 2 moles of strontium react to produce 2 moles of strontium oxide.
The molar mass of strontium (Sr) is 87.62 g/mol, and the molar mass of strontium oxide (SrO) is 119.62 g/mol. Since the molar ratio is 1:1 between strontium and strontium oxide, the mass of strontium oxide formed will be equal to the mass of strontium used.
In this case, the student added 4.93 g of strontium to the crucible. Therefore, the expected mass of strontium oxide formed will also be 4.93 g.
It's important to note that this calculation assumes that the reaction proceeds to completion, meaning that all of the strontium reacts with oxygen. In actual laboratory conditions, the yield of the reaction may be less than 100% due to factors such as incomplete reaction, side reactions, or product loss.
For more such questsion on balanced chemical equation visit:
https://brainly.com/question/11904811
#SPJ8
Please someone answer this asap

Answers
Answer:
b or c but I would just pick c
Answer:
C
Explanation:
Sun is an energy source not a matter.
The chemical on this list which is not a humectant is: O sorbitol O glycerol O lanolin O methyl O alcohol
Answers
Option (e) is correct. Alcohol is not a humectant because it binds to water and dehydrates the body.
Humectant is a substance which is used to keep things moist. A humectant attracts and retains the moisture in the air nearby via absorption. Sorbitol is a naturally occurring polyol. It is widely used in the food industry as a humectant. Sorbitol is a good humectants. Glycerol is a humectant which is most often derived naturally from vegetable oils. Humectants work to preserve other skincare ingredients and retain moisture by drawing water from the surface of your skin down into the deeper skin layers. Lanolin oil softens the skin and is a good humectant making it ideal for use in you skin care products. Methyl is an extremely effective humectant for both rinse off and leave on products. It is recommended for use in skin care products including lotions and creams.
To learn more about Humectant please visit:
https://brainly.com/question/947751
#SPJ4
a) Percy's goal is to create 22g of oxygen gas in one hour. How many moles of oxygen gas does it need to produce? Round to the nearest tenth (1 decimal place). b) Based on the molar ratios found in Step 2 (which is 2:1 ratio), how many moles of carbon dioxide must be reacted to create the oxygen required. Round to the nearest tenth.
Answers
Explanation:
a) We have to find the number of moles of oxygen gas that are present in 22 g of it. To convert from grams to moles we usually use the molar mass. To find the molar mass of oxygen gas we need the atomic mass of O.
atomic mass of O = 16.00 amu
molar mass of O = 16.00 g/mol
molar mass of O₂ = 2 * 16.00 g/mol
molar mass of O₂ = 32.00 g/mol
moles of O₂ = 22 g * 1 mol/(32.00 g)
moles of O₂ = 0.6875 moles = 0.7 moles
b) 2 CO₂ --> 2 CO + O₂
According to the coefficients of the reaction 2 moles of CO₂ will produce 2 moles of CO and 1 mol of O₂. Then the molar ratio between CO₂ and O₂ is 2:1. We will use that ratio to find the number of moles of CO₂ that are required to produce 0.7 moles of O₂.
2 moles of CO₂ : 1 mol of O₂
moles of CO₂ = 0.7 moles of O₂ * 2 moles of CO₂/(1 mol of O₂)
moles of CO₂ = 1.4 moles
Answer:
a) Percy has to produce 0.7 moles of oxygen gas.
b) 1.4 moles of carbon dioxide are required.
What is different about beaker 4 compared to beaker 1
Answers
Answer:
Graduated cylinder vs Beaker
Both graduated cylinders and beakers are pieces of laboratory glassware that have a specific function. Graduated cylinders typically are more accurate at reading the volumes of the liquid inside. Beakers are better for stirring and mixing liquids
Explanation:
scientist wants to use a model to help present the results of his detailed scientific investigation.
Why would a model be useful?
because the model makes the concepts easier to understand
because the model is easy to put together and to use
because the model prevents other scientists from asking questions
because the model requires the audience to pay full attention to it
Answers
Answer: A model would be useful because the model makes the concepts easier to understand.
Explanation:
Models are helpful tools in science education that can be used to enhance explanations, spark discussion, make predictions, provide visual representations of abstract concepts, and create mental models.
q1:
If a student over titrated the metal carbonate solution sample, how would it affect the calculated molecular mass of the metal carbonate? EXPLAIN YOUR ANSWER based on the calculations.
question 2 is attached

Answers
The mass of the barium carbonate is 0.286 g.
What is titration?The term titration refers to the manner by which we could be able to obtain the volume of a solution and then use it to estimate the amount of the analyte that is present in the reaction. When we carry out a back titration, we are essentially trying to titrate the excess that is left over.
Now we have the fact that the reaction reaction the sodium hydroxide and the excess hydrochloric acid during the back titration yields a 1:1 reaction. Thus the amount of HCl left over is;
22.48/1000 L * 0.1082
= 0.0024 moles
The amount of HCl used in the first reaction is;
25/1000 L * 0.2120 M
= 0.0053 moles
Amount of used HCl = 0.0053 moles - 0.0024 moles = 0.0029 moles
Given that;
1 mole of barium carbonate reacts with 2 moles of acid
x moles of barium carbonate reacts with 0.0029 moles of acid
x = 1 mole * 0.0029 moles/ 2moles
x = 0.00145 moles
Mass of barium carbonate = 0.00145 moles * 197.33 g/mol = 0.286 g
Learn more about titration:https://brainly.com/question/2728613
#SPJ1
A typical home instant pot has an internal volume of 6.5 Liters. If the steam inside an instant pot with an internal pressure of 191.0 kPa and a temperature of 750.0 K was released into the atmosphere at 101.3 kPa and 298K, what volume with the steam occupy in the atmosphere?
Answers
We can use the combined gas law to solve this problem.
Combined gas law: P1 V1 / T1 = P2 V2 / T2
(191.0 kPa)(6.5 L) / (750.0 K) = (101.3 kPa)(V2) / (298 K)
Now we solve for V2;
1241.5 / 750 = 101.3 / 298
Now we cross-multiply;
369967 = 75975x
Divide by 75975 on each side;
369967 / 75975 = 75975x / 75975
Round our answer to sig figs;
x (aka V2) = 4.8696 L is the volume occupied by the steam in the atmosphere
What inference can be drawn from the graph? a graph of reactant and product concentration vs. time; a red curve shows the reactant decreasing and a blue curve shows the product concentration increasing over time; the two curves do not intersect; the chemical equation shows A + B reacting to produce C + D A. The reaction between A and B is reactant favored. B. The amount of product is greater than the amount of reactant. C. The reaction uses all its reactants to form products. D. The reaction reaches equilibrium at 1.5 seconds. E. The amount of reactants is highest at equilibrium.

Answers
The reaction between A and B is reactant favored. This inference can be drawn from the graph.
Chemical reactions occur all around us, including in our body's digestion of food and the creation of the sunlight's light. Understanding both chemical and physical alterations is crucial before starting any chemical reactions. The best illustration of a chemical and physical alteration is a burning candle. The reaction between A and B is reactant favored. This inference can be drawn from the graph.
Therefore, the correct option is option A.
To know more about Chemical reaction, here:
https://brainly.com/question/29039149
#SPJ1
areas and organisms where carbon is stored
Answers
Answer:
Carbon is stored on our planet in the following major sinks (1) as organic molecules in living and dead organisms found in the biosphere; (2) as the gas carbon dioxide in the atmosphere; (3) as organic matter in soils; (4) in the lithosphere as fossil fuels and sedimentary rock deposits such as limestone, dolomite
Which of the following is NOT a "weak" interaction?
a. hydrogen bonds b. van der Waals forces c. disulfide bonds d. ionic interactions e. hydrophobic interactions
Answers
Answer:
D.ionic interaction is a strong interaction
When atoms come close enough to one another, an ionic connection is created. Atoms can interact with one another ionically, and this distance is greater than the bonding distance. Therefore, option D is correct.
What is ionic interaction ?The main interaction taking place in ionic compounds is ionic bonding, a form of chemical bonding that includes the electrostatic attraction between two atoms or ions with dramatically differing electronegativities.
Ions with opposing charges are drawn to one another through ion-ion interactions. They are also known as ionic bonds, because they are what keep ionic compounds together. opposing charges attract each other whereas like charges repel each other.
The whole transfer of valence electrons between atoms is referred to as an ionic bond. It is a kind of chemical connection that produces two ions with opposing charges. In ionic bonding, the nonmetal receives the electrons to become a negatively charged anion while the metal gives them up to become a positively charged cation.
Thus, option D is correct.
To learn more about ionic interaction follow the link;
https://brainly.com/question/4082308
#SPJ5
A galvanic cell consists of a Mg electrode in a 1 M Mg(NO3)2 solution and another metal electrode X in a 1 M X(NO3)2 solution.
The galvanic cell has an E°cell value of 1.61 V. Which of the following elements fits the identity of X. (Use table table 18.1)
Select one:
a.
Pb
b.
Zn
c.
Ni
d.
Fe
e.
Mn
Answers
Answer:
To determine the identity of metal X, we need to compare the standard reduction potentials of the possible metals with the standard reduction potential of the Mg half-reaction.
From Table 18.1, we can find the standard reduction potentials for each of the metals listed:
Pb: -0.13 V
Zn: -0.76 V
Ni: -0.25 V
Fe: -0.44 V
Mn: -1.18 V
The reduction half-reaction for the Mg electrode is:
Mg2+ + 2e- → Mg E° = -2.37 V
The overall reaction for the galvanic cell is:
Mg(s) + X2+(aq) → Mg2+(aq) + X(s)
The standard cell potential is given by:
E°cell = E°(cathode) - E°(anode)
where the cathode is the reduction half-reaction and the anode is the oxidation half-reaction.
Substituting the given values, we get:
1.61 V = E°(X2+/X) - (-2.37 V)
Simplifying, we get:
E°(X2+/X) = 1.61 V + 2.37 V = 3.98 V
Comparing E°(X2+/X) with the standard reduction potentials in Table 18.1, we see that only zinc (Zn) has a reduction potential that is more negative than 3.98 V. Therefore, the metal X is zinc (Zn).
Therefore, the answer is (b) Zn.
What is the zonecreated if force of separation occurs?
Answers
Calculate the molarity of the solution in a flask that contains 2.50 moles of potassium sulfate in 125 mL of solution.
Answers
Answer:
20M or 20mol/L
Explanation:
molarity = moles/volume (in Liters)
= 2.5/0.125L
=20M or 20 mol/L
