Amines are ________. brønsted-lowry bases brønsted-lowry acids neutral in water solution unreactive
Answers
Answer:
A.) Brønsted-Lowry bases
Explanation:
Amines have a lone pair of electrons.
Brønsted-Lowry bases donate a lone pair of electrons in exchange for a hydrogen ion.
Therefore, if exposed to an acid, amines will give up electrons in order to bond with a hydrogen. This makes them Brønsted-Lowry bases.
Related Questions
There are a couple sources that claim the answer is D, but I am having trouble finding out why that is.

Answers
The approximate temperature of the H₂O after thermal equilibrium was reached is 23.4°C (option d).
StepsThe amount of heat absorbed by the water is given as 300.0 J. The mass of water is 50.0 g and the specific heat capacity of water is 4.2 J/g°C. Let's use the equation:
Q = m * c * ΔT
where Q is the heat absorbed, m is the mass of the water, c is the specific heat capacity of water, and ΔT is the change in temperature.
Substituting the given values, we get:
300.0 J = 50.0 g * 4.2 J/g°C * ΔT
Solving for ΔT, we get:
ΔT = 300.0 J / (50.0 g * 4.2 J/g°C) = 1.43°C
The final temperature of the water can be calculated by adding the change in temperature to the initial temperature:
Final temperature = 22.0°C + 1.43°C ≈ 23.4°C
Therefore, the approximate temperature of the H₂O after thermal equilibrium was reached is 23.4°C (option d).
learn more about thermal equilibrium here
https://brainly.com/question/14556352
#SPJ1
Help please the second question. I really need help guys

Answers
Answer:
106g/mol
Explanation:
Mass of Na=2×23=46
Mass of C=12
Mass of O=3×16=48
Total mass of Na2CO3=106g/mol
n E Which is the electron configuration for zinc? O 1s22s22p3s23p64s23d8 O 1s22s22p 3s 3p64s+3d10 01s 2s 2p 3s 23p64523d10 0 1522s 2p 3s23p64533d10
Answers
The electronic configuration is used to explain the orbitals of an atom and it helps to determine the physical and chemical properties of the elements. The electronic configuration of zinc is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰. The correct option is C.
What is electronic configuration?The distribution of electrons in the atomic orbitals is given by the electronic configuration. It is a standard notation in which all the electrons holding atomic subshells are arranged in a sequence. Each element has a unique electronic configuration.
The electronic configuration of an element can be written in two ways, in standard notation, and in condensed form. In the case of elements with larger atomic numbers, the electronic configuration becomes lengthy in standard notation. So in such cases condensed form is generally used.
In condensed form electronic configuration of zinc is [Ar] 3d¹⁰4s² and in standard form it is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰.
Thus the correct option is C.
To know more about electronic configuration, visit;
https://brainly.com/question/19589767
#SPJ7
TRUE/FALSE.When a scientific theory has been tested and proved by the scientific community, it becomes a law.
Answers
The given statement "When a scientific theory has been tested and proved by the scientific community, it becomes a law" is false because a scientific theory is a well-substantiated explanation of some aspect of the natural world that is based on empirical observation, testing, and experimentation.
It is a widely accepted explanation that has been repeatedly tested and validated by multiple researchers in the scientific community over time. A scientific theory is never conclusively proven, but it is constantly tested and modified as new evidence and data emerge over time. In other words, a scientific theory is a comprehensive explanation of an observed phenomenon that has not been disproven or invalidated by testing or experimentation.
A scientific law, on the other hand, is a concise statement that describes a fundamental principle of nature that has been consistently observed to be true in every observed instance. A scientific law is a mathematical statement that accurately predicts a natural phenomenon based on empirical observations and data. Scientific laws are also considered to be widely accepted, but unlike scientific theories, they do not attempt to explain the underlying mechanisms or causes of natural phenomena.
To summarize, a scientific theory and a scientific law are not the same thing. A scientific theory is an explanation of a natural phenomenon that has been tested and validated over time, while a scientific law is a concise statement that describes a fundamental principle of nature that has been consistently observed to be true.
Therefore, when a scientific theory has been tested and proved by the scientific community, it does not become a law, as they are two different concepts with different meanings.
Learn more about scientific theory at https://brainly.com/question/22992470
#SPJ11
When referring to immunity, what does the term innate imply?
the mechanism will provide defense against many different types of pathogens
the mechanism will develop based upon exposure to specific pathogens
the mechanism will be built-in and present at birth
the mechanism will be acquired over an individual's lifetime
Answers
Answer:
vVv
Explanation:
innate means "inborn or natural", meaning they were born with it. if somewhat is born with an inate immunity, it means it was passed through genetics aka heredity
Compare how entropy changes for the following two systems:
System A: Two gases mix when the valve separating two containers is opened.
System B: A solid powder decomposes to form a solid product and a gas product.
Answers
The measure of the randomness of the system is the change in the entropy. The mixing of two gases and decomposition of the solid will increase the entropy.
What is entropy?Entropy is the disorderliness and the randomness of the system when the thermal energy is not present in a sufficient amount to initiate the reaction. In system A, when two gases are mixed then the entropy increases as the number of gaseous molecules increases.
In system B, when a solid powder gets decomposed to form a solid product and a gaseous product the entropy increases as along the solid particles the gas is also produced.
Therefore, in both the systems the entropy increases.
Learn more about entropy here:
https://brainly.com/question/27460189
#SPJ1
a chemical that allows for communication between one cell and another is a
Answers
A chemical that allows for the communication in between the one cell and another is a neurotransmitter.
The Neurotransmitters are the chemicals the type of the messenger that is used to transmit information from the one cell to the next cell. Some of the hormones are also called as the messenger chemicals. The nerve cells that are not linked with each other and they are separated from the each other by the space known as synapses. The electric potential signal that is converted into the chemical signal through the release of the neurotransmitters.
Thus, the neurotransmitter , is the chemical that allows for the communication between one cell and another .
To learn more about cell here
https://brainly.com/question/12120725
#SPJ4
Hey I'm Chloe, Can you Help me Thank you :)
Q: how can you tell if two objects are in thermal equilibrium?
Answers
Answer:
Two objects are in thermal equilibrium if they are in close contact that allows either to gain energy from the other, but nevertheless, no net energy is transferred between them. Even when not in contact, they are in thermal equilibrium if, when they are placed in contact, no net energy is transferred between them.
Explanation:
hope this helps chloe
The specific heat of liquid H2O is 4.184 J/g°C. If 32.7 grams of water are heated from
12 °C to 77 °C, how much energy is required?
Look at picture
ASAP please will mark as brainlist don’t got much time

Answers
Answer:
8893.092J
Explanation:
energy= mass × specific heat capacity of water × change in temperature
=32.7×4.184×(77-12)
=8893.092J
A rigid container holds hydrogen gas ata pressure of 3.5 atm and a temperature of 50C. what will the pressure be if the temperature is lowered to -50C? express your answer to two significant and include the appropriate units. Pf=?
Answers
The final pressure of the hydrogen gas is approximately 2.41 atm when the temperature is lowered to -50°C.
In this problem, we are given the initial pressure and temperature of hydrogen gas inside a rigid container and asked to determine the final pressure of the gas if the temperature is lowered to a certain value.
To solve this problem, we will use the combined gas law, which relates the pressure, volume, and temperature of a gas:
P₁V₁/T₁ = P₂V₂/T₂
where P₁, V₁, and T₁ represent the initial pressure, volume, and temperature, respectively, and P₂, V₂, and T₂ represent the final pressure, volume, and temperature, respectively.
We are given that the initial pressure, P₁, is 3.5 atm and the initial temperature, T₁, is 50°C. We can convert the temperature to Kelvin by adding 273.15:
T₁ = 50°C + 273.15 = 323.15 K
We are also given the final temperature, T₂, which is -50°C. We can convert this to Kelvin as well:
T₂ = -50°C + 273.15 = 223.15 K
We want to solve for the final pressure, P₂. We can rearrange the combined gas law to solve for P₂:
P₂ = (P₁ × T₂ × V₁) / (T₁ × V₂)
Since the container is rigid, the volume does not change, so V₁ = V₂. Thus, we can simplify the equation to:
P₂ = (P₁ × T₂) / T₁
Substituting in the given values, we get:
P₂ = (3.5 atm × 223.15 K) / 323.15 K
P₂ ≈ 2.41 atm
Therefore, the final pressure of the hydrogen gas is approximately 2.41 atm when the temperature is lowered to -50°C.
To know more about pressure click here:
https://brainly.com/question/4578923
#SPJ11
The molarity of a NaOH solution was determined by titration with KHP. The results of five titrations were 0.1025 M, 0.1087 M, 0.1100 M, 0.1052 M, 0.0997 M. Answer the following questions based on 95% confidence level.
a) Calculate the absolute standard deviation of the concentration of NaOH.
b) Calculate the standard error of the concentration of NaOH.
c) Calculate the confidence interval of the concentration of NaOH. Report your answer with appropriate significant figures
d) If the true concentration of this NaOH solution is 0.1045 M, is the sample mean significantly different from the true concentration?
e) Another student also measured the concentration of the same NaOH solution. The result of the three titrations were 0.1028 M, 0.1012 M, 0.0983 M. Are the mean concentrations from the two students’ result similar to each other?
Answers
a) The absolute standard deviation of the concentration of NaOH is 0.0041 M.
b) The standard error of the concentration of NaOH is 0.0018 M.
c) The confidence interval of the concentration of NaOH is (0.1033 M, 0.1060 M).
d) Yes, the sample mean is significantly different from the true concentration of 0.1045 M.
e) No, the mean concentrations from the two students' results are not similar to each other.
a) To calculate the absolute standard deviation of the concentration of NaOH, we need to find the standard deviation of the given data points. Using the formula for sample standard deviation, we calculate the average deviation of each data point from the mean concentration, then square each deviation, take the average of the squared deviations, and finally, take the square root. The absolute standard deviation is the absolute value of the standard deviation.
b) The standard error of the concentration of NaOH measures the variability of the sample means from different samples. It is calculated by dividing the standard deviation by the square root of the sample size. In this case, the sample size is 5.
c) To calculate the confidence interval of the concentration of NaOH, we need to determine the margin of error using the t-distribution and the sample standard deviation. With a 95% confidence level, we use a t-value corresponding to 4 degrees of freedom (n-1) and multiply it by the standard error. The confidence interval is constructed by subtracting and adding the margin of error to the sample mean concentration.
d) To determine if the sample mean is significantly different from the true concentration, we compare the true concentration to the confidence interval. If the true concentration falls outside the confidence interval, then the sample mean is significantly different from the true concentration.
e) To assess if the mean concentrations from the two students' results are similar to each other, we can calculate the confidence intervals for each student's data. If the confidence intervals overlap or are close to each other, it suggests that the mean concentrations are similar. However, if the confidence intervals do not overlap, it indicates that the mean concentrations are likely different.
Learn more about concentration
brainly.com/question/18247103
#SPJ11
any observation must be measurable as well as...
Answers
Part C A radioisotope emits a positron to form germanium-74. Express your answer as a nuclear equation. ΑΣΦ PUCE ? 74 Ge> 32 let 74 Ga 31 Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Part D An atom of carbon-11 emits a positron. Express your answer as a nuclear equation. CFIA_ACIA - #1....DOCX readerdelen Lacr.exe Type here to search
Answers
The nuclear reaction is expressed as \(\left \ {{74} \atop {32}} \right Ge\) ⇒ \(\left \ {{74} \atop {31}} \right Ge\) + \(\left \ {{0} \atop {+1}} \right e\) and \(\left \ {{11} \atop {12}} \right C\) ⇒ \(\left \ {{11} \atop {11}} \right C\) + \(\left \ {{0} \atop {+1}} \right e\)
A proton undergoes positron decay, which results in the creation of neutrons, electron neutrinos, and positron particles. This particle has a +1 unit charge.
\(\left \ {{A} \atop {Z}} \right X\) ⇒ \(\left \ {{A} \atop {Z-1}} \right X\) + \(\left \ {{0} \atop {+1}} \right e\)
The positron decay mode is especially useful for detection and quantification using outside measurement. When a proton is converted to a neutron and a positron, or "+-particle," is simultaneously emitted from the nucleus of a neutron-deficient isotope, the decay process starts [2]. With the exception of its positively rather than negatively charged charge, the positron shares many physical characteristics with an electron. The positron that is released is slowed down by energy loss to the surrounding matter along its route before combining with an electron.
Learn more about Nuclear equation at https://brainly.com/question/29051075
#SPJ4
Which of the following(s) is/are incorrect about the convexity term of a bond:
Group of answer choices
Convexity is always positive for a plain-vanilla bond..
We can improve the estimation of a price change with regard to a change in interest rates by accounting for the convexity of the bond.
Convexity has high value when investors expect that market yields will not change much.
Answers
The correct answer is "Convexity has high value when investors expect that market yields will not change much." This statement is incorrect about the convexity term of a bond.
Convexity is the curvature of the price-yield relationship of a bond and a measure of how bond prices react to interest rate shifts.
Convexity is a term used in bond markets to describe the shape of a bond's yield curve as it changes in response to a shift in interest rates.
Bond traders use the convexity term to estimate the effect of interest rate changes on bond prices more precisely.
Bond traders use the term convexity to measure the rate of change of duration, which is a measure of a bond's interest rate sensitivity.
Convexity term and its features Convexity is always positive for a plain-vanilla bond.
We can improve the estimation of a price change with regard to a change in interest rates by accounting for the convexity of the bond.
Convexity is higher when market yields are unstable or when the bond has more extended maturity and lower coupon rates.
Thus, the correct statement about the convexity term of a bond is:
Convexity is higher when market yields are unstable or when the bond has more extended maturity and lower coupon rates.
To know more about Convexity visit;
https://brainly.com/question/31834216
#SPJ11
CaC2 + 2H2O → C2H2 + Ca(OH)2If 4.8 moles of CaC2 are consumed in this reaction, how many grams of H2O are needed?
Answers
The given reaction is already balanced, that is to say tha the number of atoms in the reactants matches the number of atoms in the products. In the reaction, we can see the relationship between CaC2 and H2O. For each mole of CaC2 two moles of H2O react.
So, if 4.8 moles of CaC2 are consumed the moles of H2O needed will be:
Mol of H2O = Mol of CaC2 x 2
Mol of H2O = 4.8 x 2 = 9.6 mol of H2O
Now, to calculate the grams of H2O we will use the following equation and the mass molar of H2O.
Mass molar of H2O =18.01 g/mol
\(\begin{gathered} \text{Mass of H2O=Mol of H2O }\times Mass\text{ molar of H2O} \\ \text{Mass of H2O = 9.6 mol }\times18.01\frac{\text{ g}}{mol} \\ \text{Mass of H2O = 172.9 g} \end{gathered}\)So, if 4.8 moles of CaC2 are consumed in this reaction, 172.9 g of H2O are needed
which type of bonding is found in all substances made up of molecules?
Answers
Answer:
Chemical bonds
Explanation:
Chemical bonds are forces that hold atoms together to make compounds or molecules. Chemical bonds include covalent, polar covalent, and ionic bonds. Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds.
What similarities are there between Beta Radiation and Alpha Radiation
Answers
The largest particle, Alpha, has the least penetrative power. Positive charge is carried by alpha particles. Two neutrons and two protons are bound together to form an alpha particle. The helium-4 nucleus was later identified as the alpha particle. Among the three types of radioactive emissions, alpha particles have the most mass. An alpha particle has approximately 8000 times the mass of a beta particle. The penetrative power of an alpha particle is reduced due to its large size.
Beta particles are negative-charged high-energy electrons or positrons. Beta particles have a higher penetrative power than alpha particles due to their smaller size.
Hope this helps and if it does, don't be afraid to give my answer a "Thanks" and maybe a Brainliest if it's correct?
use the chemical equation to answer the question 2O2 + CH4 —> ?H2O + CO2 how many molecules of water are produced in the reaction
A- 1
B- 2
C- 4
D- 8
Answers
The correct option is B-2. 2 water molecules are produced in the reaction.
According to the given chemical reaction i.e.
2O2 + CH4 —> (x)H2O + CO2 we have to find the number of molecules of water so that all the elements in the reaction get balanced out.
Carbon atoms are equal on both sides so we have to equalize hydrogen atoms first. If we have 4 atoms of hydrogen on the left side it should be equal to the right side.
If we add two molecules of H20 the equation can be balanced.
After balancing we get,
2O2 + CH4 —> 2H2O + CO2
Learn more about molecules,
https://brainly.com/question/475709
Lori is saving money to buy a new bike. She needs $120 but has only saved 60%.
How much money has she saved so far to go towards the bike?
Answers
12 x 6 is 72
The answer is 72
please answer these questions. thanksss so much!!!!

Answers
The balanced equation of magnesium and sulfuric acid is:
Mg (s) + H₂SO₄(aq) ---> MgSO₄ (aq) + H₂ (g)The student was have seen an explosive reaction if sodium was placed in water.
The order of the reactivities of the metals from most to least reactive is: lithium >> calcium >> copper
It would be difficult to determine the order of reactivity of magnesium and zinc because they show the same reaction. This can be determined by finding out which metal displaces the other from the solution of their salt.
What are reactive metals?
Reactive metals are metals that easily form positive ions by readily giving up or donating their electrons.
The reactive metals are mainly found in group 1A and group 2A of the periodic table.
Reactive metals react with water to liberate hydrogen gas.
For example: 2 Li (s) + 2 H₂O ---> 2 LiOH (aq) + H₂ (g)
Reactive metals react with dilute acids to liberate hydrogen gas and form salts.
Mg + 2 HCl (aq) ---> MgCl₂ (aq) + H₂ (g)
The rate of the reactions above decreases with a decrease in the reactivity of the reactive metals.
Learn more about reactive metals at: https://brainly.com/question/25103661
#SPJ1
What causes pressure inside a helium balloon?
OA. The helium atoms exert an electrostatic force that pushes the
surface outward.
OB. The helium atoms expand and press on the surface of the balloon.
OC. The helium atoms bounce off the surface as they move inside the
balloon.
OD. The helium atoms stick to the surface of the balloon and increase
its weight.
Answers
Pressure inside a helium balloon: The helium atoms expand and press on the surface of the balloon.
What is helium balloon?Helium balloons are a type of balloon filled with helium gas. Helium is a light, non-flammable, inert gas that is found in abundance in the atmosphere and is used to inflate balloons. Helium balloons are often used in decorations, promotions, special events, parties, and displays. They come in a variety of shapes, sizes, and colors, and can be filled with helium and released into the atmosphere, creating a festive atmosphere. Helium balloons are also used in scientific experiments, such as measuring wind speeds, studying atmospheric pressure and air movement, and measuring the temperature of the atmosphere.
To learn more about helium balloon
https://brainly.com/question/29311706
#SPJ1
ILL MARK BRAINLIEST NEED HELP ASAP
THE OTHER TWO ANSWER CHOICES WERE
20
40
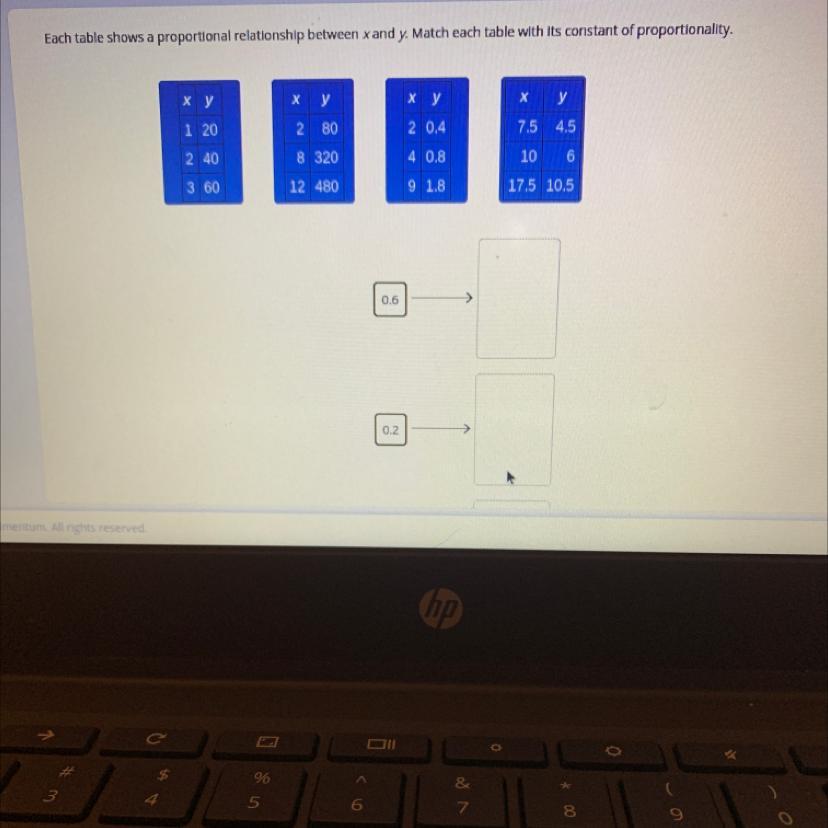
Answers
the second table goes with 40
the third table goes with .2
the fourth table goes with .6
hope this helps!!
Where does reduction occur in an electrochemical cell?
Answers
Answer:
The cathode is the electrode where reduction takes place.
so
reduction occur in Cathode in an electrochemical cell.
Answer: Cathode
Explanation:
.
is 2.71 greater then 8?
Answers
Answer:
no
Explanation:To help you know the difference change it into a percentage, by moving the decimal two places to the right. That would give you 271% and 800% would you rather get a 271% or a 800% on a test? You would want to get an 800% because it’s a bigger number.
Compare and contrast the political system
(institutions, branches of government, electoral rules) of France
and Russia. How do they compare? What are the key distinguishing
features? What are the stre
Answers
Russia is a federation with a semi-presidential political system. The President is the head of state while the Prime Minister is the head of government. The Federal Assembly is a bicameral legislature that is made up of the State Duma (lower house) and the Federation Council (upper house).
The political system in Russia and the United States are different. In the US, it is a presidential system where the President is both the head of state and government, while in Russia, the President is the head of state while the Prime Minister is the head of government.
In the US, the Congress is made up of the Senate (upper house) and the House of Representatives (lower house) while in Russia, the Federal Assembly is made up of the State Duma (lower house) and the Federation Council (upper house).
The key distinguishing features between the political systems in Russia and the US include the role of the President, the structure of the legislature, and the nature of the judiciary. In Russia, the President has a lot of power and is able to appoint the Prime Minister and other members of the executive branch.
The judiciary is also less independent compared to that of the US. On the other hand, the US has a more balanced system of power between the three branches of government, with the judiciary being independent of the executive and legislative branches.
The strengths of the political system in Russia include a strong centralized government that is able to make quick decisions and a strong military. However, the lack of political pluralism and the weak judiciary system are key weaknesses of the system.
The US political system has a strong commitment to individual rights and democratic principles. However, the system is often characterized by gridlock and polarization between political parties, leading to slow decision-making and a lack of progress on important issues.
To know more about Federal Assembly here
https://brainly.com/question/28863069
#SPJ11
How do I complete this? Also how do I hypothesize them? What is the formula for the first one specifically?

Answers
The balanced equations of the given reactions are:
1. 2 Mg + O₂ ---> 2 MgO + heat + light
2. (NH₄)₂CO₃ + O₂ ---> 2 NH₃ + CO₂ + H₂O
3. Fe + CuSO₄ ---> FeSO₄ + Cu
4. C₂H₅OH + 3 O₂ ---> 2 CO₂ + 3 H₂O + heat + light
5. Pb(NO₃)₂ + 2 KI ---> 2 KNO₃ + PbI
What are the balanced equations of the given reactions?The balanced equations of the given reactions are derived from the hypothesis of the product formed.
1. When magnesium metal is heated in air, it will react with oxygen to produce magnesium oxide and release heat and light.
2 Mg + O₂ ---> 2 MgO + heat + light
2. When ammonium carbonate is heated in air, it will decompose into ammonia, carbon dioxide, and water vapor, and release a pungent odor.
(NH₄)₂CO₃ + O₂ ---> 2 NH₃ + CO₂ + H₂O
3. When steel wool (iron) is placed into a solution of copper (II) sulfate, a single displacement reaction will occur where iron will replace copper in the compound, forming iron (II) sulfate and copper metal.
Fe + CuSO₄ ---> FeSO₄ + Cu
4. When ethanol (C₂H₅OH) is lit with a lighter, it will burn and produce carbon dioxide and water vapor, and release heat and light.
C₂H₅OH + 3 O₂ ---> 2 CO₂ + 3 H₂O + heat + light
5. When a solution of lead (II) nitrate is mixed with a solution of potassium iodide, a double displacement reaction will occur where lead will replace potassium in the compound, forming lead iodide and potassium nitrate, which will precipitate out as a yellow solid.
Pb(NO₃)₂ + 2 KI ---> 2 KNO₃ + PbI
Learn more about balanced equations at: https://brainly.com/question/26694427
#SPJ1
Sort the following elements according to how they are most likely to be found in nature. Au, V, Cd, Al, Cu
Answers
The elements can be in terms of their likelihood of being found in nature as follows: Aluminum (Al) > Copper (Cu) > Gold (Au) > Cadmium > Vanadium (V). Aluminum is the most abundant metal in the Earth, making up approximately 8% of its composition.
Copper is the next most likely element to be found in nature. While not as abundant as aluminum, it is still relatively common. Copper occurs naturally in various minerals, including copper sulfides and copper oxides. It is often found with other metals in deposits. Gold is often associated with geological processes such as hydrothermal activity or erosion. Due to its scarcity and inherent value, gold has been treasured and used for ornamental and monetary purposes throughout history.
Learn more about elements here.
https://brainly.com/question/2050606
#SPJ1
what is transitional elements
Answers
Answer:
any of the set of metallic elements occupying a central block (Groups IVB–VIII, IB, and IIB, or 4–12) in the periodic table, e.g., iron, manganese, chromium, and copper. Chemically they show variable valence and a strong tendency to form coordination compounds, and many of their compounds are colored.
Explanation:
What is the correct formula for Triphosphorous hexachloride?
Answers
Answer:
P3
Explanation:
Im pretty sure hope this helps
Consider the chemical equations shown here.
P4(s)+3O2(g)--->P4O6(s) ΔH1 = -1,640.1 kJ
P4O10(s) → P4(s) + 5O2(g) ΔH2 = 2,940.1 kJ
What is the overall enthalpy of reaction for the equation shown below?
Round the answer to the nearest whole number.
P4O6(s) + 2O2(g) --->P4O10(s)
Answers
Answer:
-1300. kJ
Explanation:
We have two equations:
1. P₄(s) +3O₂(g) ⟶ P₄O₆(s); ΔH₁ = -1640.1 kJ
2. P₄O₁₀(s) ⟶ P₄(s) + 5O₂(g); ΔH₂ = 2940.1 kJ
From these, we must devise the target equation:
3. P₄O₆(s) + 2O₂(g) ⟶ P₄O₁₀(s); ΔH = ?
The target equation has P₄O₆(s) on the left, so you reverse Equation 1.
When you reverse an equation, you reverse the sign of its ΔH.
4. P₄O₆(s) ⟶ P₄(s) +3O₂(g); ΔH₁ = 1640.1 kJ
Equation 4 has P₄ on the right. That is not in the target equation.
You need an equation with P₄ on the left, so you reverse Equation 2.
5. P₄(s) + 5O₂(g) ⟶ P₄O₁₀(s); ΔH₂ = -2940.1 kJ
Now, you add equations 4 and 5, cancelling species that appear on opposite sides of the reaction arrows.
When you add equations, you add their ΔH values.
You get the target equation 3:
4. P₄O₆(s) ⟶ P₄(s) + 3O₂(g); ΔH₁ = 1640.1 kJ
5. P₄(s) + 2(5)O₂(g) ⟶ P₄O₁₀(s); ΔH₂ = -2940.1 kJ
3. P₄O₆(s) + 2O₂(g) ⟶ P₄O₁₀(s); ΔH = -1300. kJ
ΔH for the reaction is -1300. kJ