Arrange the compounds in order of decreasing boiling point.Rank the compounds from the highest to lowest boiling point.(CH3)2(CH3CH2)NCH3CH2CH2CH2NH2CH3CH2CH2CH2OH
Answers
The order of decreasing boiling point for the given compounds is:
CH₃CH₂CH₂CH₂NH₂ > (CH₃)₂(CH₃CH₂)NCH₃CH₂CH₂CH₂OH > CH₃CH₂CH₂CH₂OH
The first compound, CH₃CH₂CH₂CH₂NH₂, has the highest boiling point due to the stronger intermolecular forces caused by the dipole-dipole interactions between the nitrogen atom and the hydrogen atoms of the amine group.
The second compound, (CH₃)₂(CH₃CH₂)NCH₃CH₂CH₂CH₂OH, has a lower boiling point than the first compound because the presence of an oxygen atom in the alcohol group reduces the strength of the intermolecular forces.
The third compound, CH₃CH₂CH₂CH₂OH, has the lowest boiling point due to the lowest number of polar groups.
To know more about stronger intermolecular forces click on below link:
https://brainly.com/question/13479228#
#SPJ11
Related Questions
which of the following samples has the most moles of the compound? a) 163.0 g of fe2o3 b) 75.0 g of cas c) 150.0 g of bao d) all of the above have the same moles. e) impossible to determine unless the density of each compound is known.
Answers
The samples that has the most moles of the compound is option B which is 75.0g
Moles calculation .
To determine which sample has the most moles of the compound, we need to calculate the number of moles of each compound using its molar mass.
a) Fe2O3:
Molar mass of Fe2O3 = 2(55.85 g/mol of Fe) + 3(16.00 g/mol of O) = 159.70 g/mol
Number of moles of Fe2O3 = 163.0 g / 159.70 g/mol = 1.02 mol
b) CaS:
Molar mass of CaS = 40.08 g/mol of Ca + 32.06 g/mol of S = 72.14 g/mol
Number of moles of CaS = 75.0 g / 72.14 g/mol = 1.04 mol
Therefore, sample b) (75.0 g of CaS) has the most moles of the compound, with 1.04 moles. Sample a) (163.0 g of Fe2O3) has 1.02 moles and sample c) (150.0 g of BaO) has 0.98 moles.
So, the correct answer is b.
Learn more about moles below.
https://brainly.com/question/15356425
#SPJ1
a 76.8 lb 76.8 lb child has a streptococcus infection. amoxicillin is prescribed at a dosage of 45 mg per kg 45 mg per kg of body weight per day given b.i.d.
Answers
The child needs 1,569.25 mg of amoxicillin per day, with 784.63 mg of amoxicillin per dose.
Streptococcus infections are commonly treated with amoxicillin, a broad-spectrum antibiotic.
Amoxicillin is effective against many different types of bacteria, including Streptococcus bacteria.
When a child has a streptococcus infection, amoxicillin may be prescribed at a dosage of 45 mg per kg of body weight per day given b.i.d.
In this case, a 76.8 lb child would be given 1,385.28 mg of amoxicillin per day, divided into two equal doses, for a total of 692.64 mg per dose.
Amoxicillin is a penicillin antibiotic used to treat infections caused by bacteria.
It is effective against many different types of bacteria, including streptococcus bacteria.
The required dosage of amoxicillin for a child is determined by their body weight and the extent of the infection they are experiencing.
In this case, the child weighs 76.8 lbs, which is equivalent to 34.85 kg.
The dosage of amoxicillin is 45 mg per kg of body weight per day, so the child needs 1,569.25 mg of amoxicillin per day.
This dosage is divided into two equal doses, so the child needs 784.63 mg of amoxicillin per dose.
Since amoxicillin is often taken orally, this dosage can be provided in the form of a tablet, suspension, or chewable tablet.
The duration of amoxicillin treatment will depend on the severity of the infection and the response of the child to the treatment. Generally, amoxicillin treatment lasts for 10 to 14 days.
The child should continue taking amoxicillin for the full prescribed course, even if they start feeling better before the treatment is completed.
Learn more about amoxicillin at: https://brainly.com/question/15701486
#SPJ11
it is desired to inflate a baggie with a volume of 836 milliliters by filling it with nitrogen gas at a pressure of 1.05 atm and a temperature of 301 k. how many grams of n2 gas are needed
Answers
To inflate the baggie with a volume of 836 milliliters using nitrogen gas at a pressure of 1.05 atm and a temperature of 301 K, we can apply the ideal gas law. Using the equation PV = nRT and rearranging it to solve for the number of moles (n), we find that n = PV / RT, which yields approximately 0.08757 moles of nitrogen gas. By multiplying this value by the molar mass of nitrogen (28.02 g/mol for N₂), we can determine the mass of nitrogen gas needed, which comes out to be approximately 2.453 grams. Thus, around 2.453 grams of nitrogen gas are required to inflate the baggie to the desired volume.
To determine the mass of nitrogen gas needed to inflate the baggie, we can use the ideal gas law equation:
PV = nRT
Where:
P = Pressure = 1.05 atm
V = Volume = 836 mL = 0.836 L (converted from milliliters to liters)
n = Number of moles of gas (what we want to find)
R = Ideal gas constant = 0.0821 L·atm/(mol·K)
T = Temperature = 301 K
Rearranging the equation, we can solve for the number of moles (n):
n = PV / RT
n = (1.05 atm * 0.836 L) / (0.0821 L·atm/(mol·K) * 301 K)
n = 0.08757 mol (rounded to 5 decimal places)
Now, we can calculate the mass of nitrogen gas using the molar mass of nitrogen (N₂):
Molar mass of N₂ = 2 * atomic mass of nitrogen (N)
Atomic mass of N = 14.01 g/mol
Molar mass of N₂ = 2 * 14.01 g/mol
Molar mass of N₂ = 28.02 g/mol
Finally, we can calculate the mass of nitrogen gas (m) using the number of moles (n) and the molar mass (M):
m = n * M
m = 0.08757 mol * 28.02 g/mol
m = 2.453 g (rounded to 3 decimal places)
Therefore, approximately 2.453 grams of nitrogen gas are needed to inflate the baggie.
To know more about Molar mass, visit:
https://brainly.com/question/3658475
#SPJ11
During a phase change, such as melting or boiling, the kinetic energy __. Highlight correct answer
Answers
Answer: During a phase change, such as melting or boiling, the kinetic energy increases.
Explanation:
Kinetic energy is the energy obtained by the molecules of a substance due to their motion.
When phase change such as melting or boiling takes place then it means heat is absorbed by the substance. As a result, their molecules more even more rapidly from one place to another due to which more number of collisions take place.
Hence, a change in phase of substance occurs as kinetic energy increases in melting or boiling process.
Thus, we can conclude that during a phase change, such as melting or boiling, the kinetic energy increases.
27.5 cm³ of a solution of NaOH neutralizes 25.0cm³ of 0.5 MHCL solution. Calculate the
concentration of NaOH in
b. gdm
a. Moldm-3
Answers
a)The concentration of NaOH in g/dm³ is approximately 18.18 g/dm³, and b)The concentration in mol/dm³ is approximately 0.4545 mol/dm³.
a)To calculate the concentration of NaOH in g/dm³ (grams per cubic decimeter) and mol/dm³ (moles per cubic decimeter), we need to know the amount of NaOH used in the reaction and the volume of the NaOH solution.
From the given information, we have:
Volume of NaOH solution = 27.5 cm³
Volume of HCl solution = 25.0 cm³
Molarity of HCl solution = 0.5 M
Since the reaction between NaOH and HCl is a 1:1 stoichiometric ratio, the moles of NaOH used can be determined from the moles of HCl used:
Moles of HCl = Molarity × Volume = 0.5 M × 25.0 cm³ = 12.5 mmol (millimoles)
Since the moles of NaOH used is also equal to the moles of HCl, we have:
Moles of NaOH = 12.5 mmol
b)To calculate the concentration of NaOH in g/dm³, we need to convert moles to grams using the molar mass of NaOH, which is approximately 40 g/mol:
Mass of NaOH = Moles × Molar mass = 12.5 mmol × 40 g/mol = 500 g
Now, we can calculate the concentration in g/dm³:
Concentration of NaOH (g/dm³) = Mass of NaOH / Volume of NaOH solution
= 500 g / 27.5 cm³
≈ 18.18 g/dm³
To calculate the concentration of NaOH in mol/dm³, we can use the same approach:
Concentration of NaOH (mol/dm³) = Moles of NaOH / Volume of NaOH solution
= 12.5 mmol / 27.5 cm³
≈ 0.4545 mol/dm³
Therefore, the concentration of NaOH in g/dm³ is approximately 18.18 g/dm³, and the concentration in mol/dm³ is approximately 0.4545 mol/dm³.
Know more about concentration here:
https://brainly.com/question/28464162
#SPJ8
give a reason for the following questions:
1. Silver bromide is stored in a brown colour thick glass bottle
Answers
Silver bromide is stored in a brown colour thick glass bottle in order to regulate the amount of light that enters it.
What is silver bromide used for?Silver bromide is an inorganic compound; the silver salt of hydrobromic acid, AgBr, sometimes used in photographic papers.
Silver bromide is a photosensitive chemical compound that easily undergoes photolytic breakdown. It quickly interacts with light and loses its characteristic by generating bromine gas and silver.
Silver bromide tends to decompose when light falls on it. In order to prevent this, dark colored bottles are used as they restrict the amount of light entering the bottle.
Learn more about silver bromide at: https://brainly.com/question/26743063
#SPJ1
) What mass of potassium nitrate would be needed to
produce a saturated solution of potassium nitrate in
50 grams of water at 313 K?
Answers
Answer:
the mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K is
\(31g\)
Explanation:
To determine the mass of potassium nitrate needed to
produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?, then it implies that we need to determine the
solubility of KNO3 is 62g KNO3 in 100g H2O at 313K. And this simply the amount of solute that when it dissolved in that water, then the water will not be able to take more solute again which means it has been saturated.that is the maximum quantity that the water can take at 313K.
If the solubility of KNO3 is 62g KNO3 in 100g H2O at temperature 313K
Then 50 g of water contains potassium nitrate = (62/100 X 50) at 313k = 31g
Therefore, the mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K is
31g
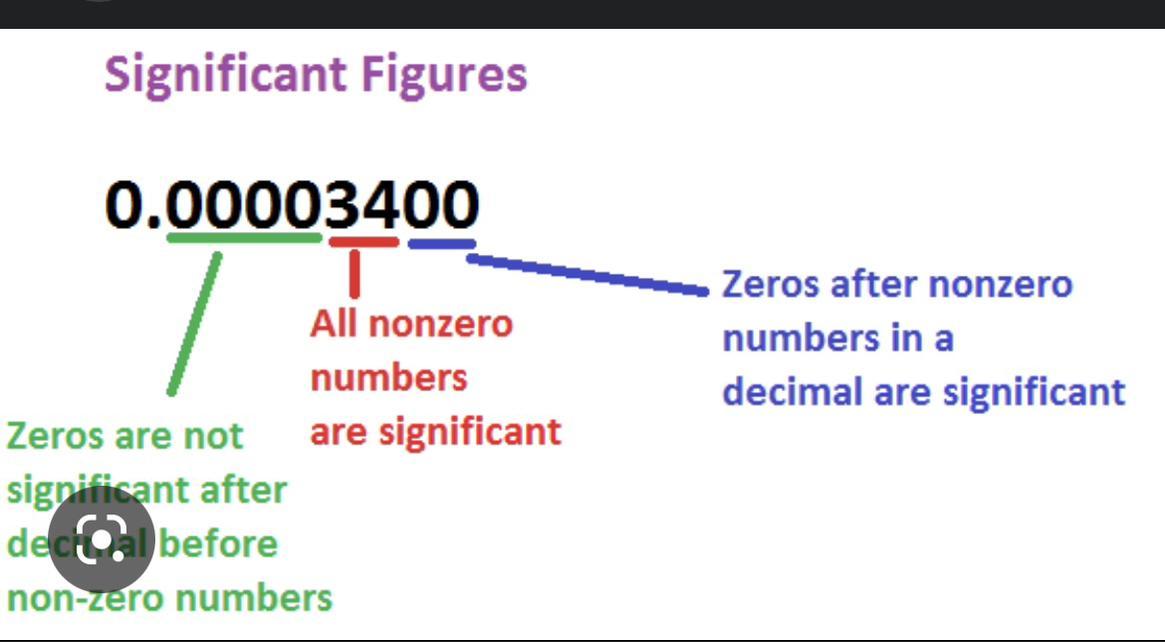
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
11. Group 2 on the periodic table corresponds to what family of elements?
Answers
Answer:
Alkaline earth metals
Explanation:
Unit Test Review
Active
1
2
3
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to
another?
O The volume and the shape stay the same.
O The volume increases to fill the new container, but the shape stays the same.
O The volume stays the same, but the shape changes to fit the new container
O The volume and the shape change to fill the new container
Answers
Answer:
the volume stays the same, but the shape changes to fit the new container
Explanation:
because the same amount/volume of liquid is being placed in a new container, the new container has a different shape to the old, thus changing the liquids shape but not volume.
When 3.0 mol O_2(g) is heated at a constant pressure of 3.25 atm, its temperature increases from 260 K to 285 K. Given that the molar heat capacity of O_2 at constant pressure is 29.4 J K^-1 mol^-1, calculate q, Delta H, and Delta U.
Answers
The values are- q= 735 J, Delta H = 735 J, Delta U = 735 J.
The change in temperature (Delta T) of 3.0 mol of O_2 can be calculated as 285 K - 260 K = 25 K. The amount of heat absorbed (q) can be found using the equation q = Cp * Delta T, where Cp is the molar heat capacity of O_2 at constant pressure. Plugging in the values, we get q = 29.4 J K^-1 mol^-1 * 25 K = 735 J.
The change in enthalpy (Delta H) can be calculated using the equation Delta H = q + w, where w is the work done by the system. In this case, the pressure is constant, so the work done is 0 J, and Delta H = q = 735 J.
The change in internal energy (Delta U) can be calculated using the equation Delta U = Delta H - P * Delta V, where Delta V is the change in volume and P is the pressure. Since the pressure is constant, the change in volume is 0, and Delta U = Delta H = 735 J.
To read more about enthalpy, Visit-
https://brainly.com/question/13996238
#SPJ11
colors seen on the surface of gasoline are primarily the result of
Answers
The colors seen on the surface of gasoline are primarily the result of a phenomenon called thin-film interference. This occurs when light waves reflect off both the top and bottom surfaces of the thin gasoline layer, causing the reflected waves to interfere with each other.
The resulting chemical colors depend on the thickness of the gasoline film and the angle at which the light is observed.
The colors seen on the surface of gasoline are primarily the result of thin-film interference. When light enters the surface of gasoline, some of it is reflected off the top of the liquid, while some of it enters the liquid and reflects off the bottom surface of the liquid. These two waves of light can interfere with each other, causing some wavelengths of light to cancel out while others reinforce. This interference produces the characteristic rainbow-like colors seen on the surface of gasoline. The exact colors seen can depend on a variety of factors, including the thickness of the liquid, the angle of incidence of the light, and the composition of the gasoline.
learn more about gasoline here
https://brainly.com/question/6967896
#SPJ11
Molecules that increase the production and release of neurotransmitters are known as?
Answers
Molecules which increase neurotransmitter formation and release are termed as calcium.
The release of neurotransmitter molecules is triggered by an increase in calcium. When a neuron becomes depolarized and reaches a threshold, it sends an electrical signal called the action potential down the axon. When the action potential reaches the terminal, voltage gated calcium channels open. This causes an influx of calcium, which binds to proteins and allows the fusion of neurotransmitter-filled vesicles with the membrane. This results in the release of increased level of neurotransmitter into the synapse, where it can cause changes in the postsynaptic cell.
What are Neurotransmitters?
Neurotransmitters are chemicals that neurons release in order to communicate with other neurons or effector tissues such as muscle. There are various types of neurotransmitters, each with a unique effect on the postsynaptic cell.
Find more on neurotransmitters at : brainly.com/question/26387085
#SPJ4
What is the reason developed nations are more likely to consider solutions to environmental problems than less developed nations?
Answers
Answer:
most developed nations.....
Explanation:
have more people and more resources to fix problems
How do you test oxygen
Answers
Answer:
The glowing splint test is a test for an oxidizing gas, such as oxygen. In this test, a splint is lit, allowed to burn for a few seconds, then blown out by mouth or by shaking. Whilst the ember at the tip is still glowing hot, the splint is introduced to the gas sample that has been trapped in a vessel. Oxygen supports combustion so a good method of testing for oxygen is to take a glowing splint and place it in a sample of gas, if it re-ignites the gas is oxygen. This is a simple but effective test for oxygen.
Answer:
✧ \( \underline{ \underline{ \large{ \tt{Test \: Of \: Oxygen \: Gas}}} }: \)
To test whether the produced gas is oxygen or not , introduce a glowing matchstick in the jar containing gas. This burns with bright light. It proves that the gas in the gas jar is oxygen.When a burning magnesium ribbon is introduced inside the gas jar , it burns with dazzling light and produces white ash of magnesium oxide. This proves that the gas jar contains oxygen.ツ Hope I helped! ☃
☂ Have a wonderful day / night !♡
✎ \( \underbrace{ \overbrace{ \mathfrak{Carry \: On \: Learning}}}\)
▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁
One mole of O2 has approximately the same mass as one mole of
Answers
One mole of O2 has approximately the same mass as one mole of methanol.
Mole and mass of substancesRecall that: mole = mass/molar mass, hence, mass = mole x molar mass.
O2 has a molar mass of 32 g/mol
Using the above equation, the mass of one mole of O2 will be:
Mass of 1 mole O2 = 1 x 32 = 32 grams
Methanol has a molar mass of 32 g/mol.
Mass of 1 mole \(CH_3OH\) = 1 x 32 = 32 grams.
In other words, 1 mole of O2 will have approximately the same mass as 1 mole of CH3OH.
More on mass and mole of substances can be found here: https://brainly.com/question/21042927
#SPJ1
Write a balanced overall reaction from these unbalanced half reactions:
Cu ---------------> Cu^+2
Ag^+ -------------------> Ag
Please note: 2Ag ^+ + Cu ----------------> 2Ag + Cu^+2 is not the correct answer
Answers
Cu + 2Ag+ → Cu2+ + 2Ag The balanced chemical equation should be written as:Copper (Cu) reacts with Silver ions (Ag+) to form Copper ions (Cu2+) and silver (Ag).
The unbalanced chemical equations are:Cu → Cu2+Ag+ → AgStep 1: Balance the half-reaction for copper ions (Cu)Cu → Cu2+ + 2e-Step 2: Balance the half-reaction for silver ions (Ag+)Ag+ + e- → AgStep 3: Equate the number of electrons in both half-reactions.
The number of electrons in the two half reactions are not equal, therefore, they need to be balanced.Cu → Cu2+ + 2e-2Ag+ + 2e- → 2AgThe number of electrons is equal on both sides now.Step 4: Add the two balanced half reactions together and cancel out the electrons.Cu + 2Ag+ → Cu2+ + 2AgThis is the balanced overall reaction from the unbalanced half reactions. Therefore, the correct option is (Cu + 2Ag+ → Cu2+ + 2Ag).
To know more about Copper ions visit:
https://brainly.com/question/28944129
#SPJ11
a poorly planned crossed aldol reaction can produce how many different aldol regioisomers?
Answers
A poorly planned crossed aldol reaction can produce four different aldol regioisomers.
An aldol reaction is a method for synthesizing new carbon–carbon bonds in organic chemistry. It occurs between an enolate and a carbonyl group. In a crossed aldol reaction, the reactants come from two distinct molecules. When an aldehyde or a ketone is reacted with another carbonyl compound, a crossed aldol reaction occurs.
In this reaction, two different carbonyl compounds are combined. The nucleophilic enolate of one carbonyl compound reacts with the electrophilic carbonyl carbon of another carbonyl compound. It yields a new β-hydroxy carbonyl compound. The following are some examples of a poorly planned crossed aldol reaction: The production of aldol regioisomers is possible when the reaction is poorly planned.
To know more about regioisomers visit:
https://brainly.com/question/31434808
#SPJ11
A substance is on its fifth half-life. Which approximates the percentages of radioactive and stable isotopes, respectively? 96.9%; 3.1% 3.1%; 96.9% 12.5%; 87.5% 87.5%; 12.5% Need an answer fast
Answers
Answer:
B
Explanation:
Edge 2020
Answer:
B babeeeeeeeeeeeeee
Explanation:
Use the activity series below the predict the products of each of the following reactions. Do not worry about balancing the equations, CCl4 + Br2--? no reaction, CBr4 + Cl2
Answers
Answer:
1. a
2.b
Explanation:
In Lhasa, Tibet, the elevation is 12,000 feet. The altimeter reading in an airplane is 19.50 in Hg. This pressure is equal to A) 9.58 B) 495 C)0.651 D) 1.61 E) 23.7 torr
Answers
The pressure of the altimeter reading in an airplane and the elevation is 12,000 fee is 19.50 in Hg is 1.61 (Option D).
At higher altitudes, the atmospheric pressure decreases, and this decrease can be measured using an altimeter. The altimeter reading of 19.50 in Hg indicates a lower pressure at 12,000 feet elevation. To convert this to the standard unit of pressure, we use the equation:
Pressure in atm = Altitude factor x Standard pressure at sea level
where the altitude factor is calculated as:
Altitude factor = (Altimeter reading at altitude / Standard pressure at sea level)\(^{(1/5.257)}\)
Plugging in the given values:
Altitude factor = (19.50 / 29.92)\(^{(1/5.257)}\) = 0.593
Standard pressure at sea level is 1 atm or 760 mm Hg or 101.3 kPa.
Therefore,
Pressure in atm = 0.593 x 1 atm = 0.593 atm
Converting to other units:
Pressure in torr = 0.593 x 760 torr = 451.08 torr
Pressure in mm Hg = 0.593 x 760 mm Hg = 453.8 mm Hg
Pressure in kPa = 0.593 x 101.3 kPa = 60.4 kPa
The closest answer option is 1.61, which is the conversion factor between atm and in Hg.
Learn more about altimeter: https://brainly.com/question/13181528
#SPJ11
THIS IS A THREE PART QUESTION IF YOU CAN HELP IT WOULD BE REALLY APPRECIATED SO I DONT FAIL.
1. If I add 2.65L of water to 245 grams of sodium acetate, what is the molarity of the NaC2H3O2 solution?
A. 10.8 m
B. 1.13 m
C. 1.08 m
D. 92.4 m
2. If I add 2.65 L of water to 245 grams of sodium acetate, what is the % by mass of NaC2H3O2 in this solution?
A. 8.46%
B.1.12%
C.10.8%
D.9.25%
3. If I add 2.65 L of water to 245 grams of sodium acetate, what is the mol fraction of NaC2H3O2 in this solution?
A. 0.203
B. 0.0846
C. 0.108
D. 0.0199
Answers
Answer:
1. B = 1.13M
2. A. 8.46%
3. D = 0.0199
Explanation:
1. Molarity of a a solution = number of moles of solute/ volume of solution in L
Number of moles of solute = mass of solute/molar mass of solute
Molar mass of NaC₂H₃O₂ = 82 g/mol, mass of NaC₂H₃O₂ = 245 g
Number of moles of NaC₂H₃O₂ = 245 g / 82 g/mol = 2.988 moles
Molarity of solution = 2.988 mols/ 2.65 L = 1.13 M
2. Percentage by mass of a substance = mass of substance /mass of solution × 100%
Mass of 2.65 L of water = density × volume
Density of water = 1 Kg/L = 1000 g/L; volume of waterb= 2.65 L
Mass of water = 1000 g/L × 2.65 L = 2650 g
Mass of solution = mass of water + mass of solute = 245 + 2650 =2895 g
Percentage by mass of NaC₂H₃O₂ = 245/2895 × 100% = 8.46%
3. Mole fraction of NaC₂H₃O₂ = moles of NaC₂H₃O₂/moles of solution
Moles of water = mass /molar mass
Mass of water = 2650 g; molar mass of water = 18 g/mol
Moles of water = 2650 g / 18 g/mol = 147.222 moles
Moles of solution = moles of solute + moles of water = 147.222 + 2.988 = 150.21
Moles of NaC₂H₃O₂ =2.988 moles
Moles fraction of NaC₂H₃O₂ =2.988/150.21 = 0.0199
oxygen is estimated to make up 28.7% of the mass of ordinary matter on earth, while magnesium makes up 15.4%. estimate the ratio of the number of oxygen atoms to the number of magnesium atoms on earth. 2.82 2.88 3.22 2.73
Answers
In the given question, option a. 2.82 is the predicted ratio of the amount of oxygen atoms to the number of magnesium atoms on Earth.
Matter is anything that has mass and takes up space. It can exist in different forms, such as solid, liquid, or gas, and it is made up of tiny particles called atoms.
To estimate the ratio of the number of oxygen atoms to the number of magnesium atoms on earth, we need to compare the mass percentage of each element with their atomic masses.
The atomic mass of oxygen is 16, while the atomic mass of magnesium is 24.31.
The mass percentage of oxygen is 28.7%, so the mass of oxygen in 100 grams of ordinary matter is 28.7 grams.
The mass percentage of magnesium is 15.4%, so the mass of magnesium in 100 grams of ordinary matter is 15.4 grams.
The number of oxygen atoms in 28.7 grams of oxygen is (28.7 g / 16 g/mol) = 1.79 moles of oxygen.
The number of magnesium atoms in 15.4 grams of magnesium is (15.4 g / 24.31 g/mol) = 0.634 moles of magnesium.
The ratio of the number of oxygen atoms to the number of magnesium atoms: \(\rm \dfrac {(1.79 \ moles\ of \ oxygen)} { (0.634\ moles \ of \ magnesium) }\)
= 2.82
Therefore, the estimated ratio of the number of oxygen atoms to the number of magnesium atoms on earth is option a. 2.82.
Learn more about matter here:
https://brainly.com/question/6966886
#SPJ12
Your question is incomplete but most probably your full question was:
Oxygen is estimated to make up 28.7% of the mass of ordinary matter on earth, while magnesium makes up 15.4%. estimate the ratio of the number of oxygen atoms to the number of magnesium atoms on earth.
a. 2.82
b. 2.88
c. 3.22
d. 2.73
What is faradays first law
Answers
Answer:
“Whenever a conductor is placed in a varying magnetic field, an electromotive force is induced. Likewise, if the conductor circuit is closed, a current is induced, which is called induced current.”
Explanation:
What happens to metals as they lose thermal energy?
Answers
which of the following described the process of osmosis?
Answers
Answer:
a process which occurs when there is a difference in solute concentration between two solutions separated by a semipermeable membrane.
hope this helps
Complete and balance each of the following equations. If no reaction occurs, enter NOREACTION.
LiI(aq)+BaS(aq)→
KCl(aq)+CaS(aq)→
MnBr2(aq)+Na2CO3(aq)→
NaOH(aq)+Fe(NO3)3(aq)→
Answers
1. LiI(aq) + BaS(aq) → Li2S(aq) + BaI2(aq)
2. KCl(aq) + CaS(aq) → K2S(aq) + CaCl2(aq)
3.. MnBr2(aq) + Na2CO3(aq) → MnCO3(aq) + 2NaBr(aq)
4. NaOH(aq) + Fe(NO3)3(aq) → 3NaNO3(aq) + Fe(OH)3(s)
Let's get started finish and balance each of the chemical equations provided:
1. LiI(aq) + BaS(aq) →
In this instance, it is clear that both barium sulphide (BaS) and lithium iodide (LiI) are ionic compounds. By examining whether an ion exchange that may result in the production of a precipitate could occur, we can determine if a reaction has taken place.
We can see that the reactants contain the ions lithium (Li+) and barium (Ba2+). Barium iodide (BaI2) and lithium sulphide (Li2S) are the products of the combination of these ions:
LiI(aq) + BaS(aq) → Li2S(aq) + BaI2(aq)
2. KCl(aq) + CaS(aq) →
Similar to the preceding equation, we must determine whether an ion exchange that results in the development of a precipitate is possible.
The reactants in this instance are potassium chloride (KCl) and calcium sulphide (CaS). These ions interact to generate calcium chloride (CaCl2) and potassium sulphide (K2S):
KCl(aq) + CaS(aq) → K2S(aq) + CaCl2(aq)
3. MnBr2(aq) + Na2CO3(aq) →
Once more, we must determine whether a reaction takes place by looking at any potential ion exchange between manganese bromide (MnBr2) and sodium carbonate (Na2CO3).
These ions react to generate sodium bromide (NaBr) and manganese carbonate (MnCO3):
MnBr2(aq) + Na2CO3(aq) → MnCO3(aq) + 2NaBr(aq)
4. NaOH(aq) + Fe(NO3)3(aq) →
In this instance, the reactants are sodium hydroxide (NaOH) and iron(III) nitrate (Fe(NO3)3). They will combine to generate iron(III) hydroxide (Fe(OH)3) and sodium nitrate (NaNO3):
3NaOH(aq) + Fe(NO3)3(aq) → 3NaNO3(aq) + Fe(OH)3(s)
Therefore, the balanced equation is:
3NaOH(aq) + Fe(NO3)3(aq) → 3NaNO3(aq) + Fe(OH)3(s).
For more such questions on BaS visit:
https://brainly.com/question/28656613
#SPJ8
33,792 ft = ? miles. can you help me please
Answers
Answer:
6.4 miles
Could you please give me brainliest, I need it.
Explanation:
The penny coin was removed from circulation in Canada
In February of 2014. The United States may soon do the
same. The major reason for this move was the rising value
of copper metal. When copper’s value increased, pennies
were produced as a zinc slug with a thin layer of copper
plated over top. Zinc reacts readily with hydrochloric acid,
while copper does not. A triangular file is used to nick the
edge of a penny to expose the zinc slug below the layer
of copper. The zinc reacts with the acid releasing bubbles
from the nicked area until nothing remains but a thin shell
of copper. If 0.948 L of hydrogen gas is collected over water
at 20.0°C and a total pressure of 752 mm Hg, determine the
percentage by mass of the copper in the 2.586 g penny.
Answers
As a result, the dry gas volume at STP is 45.58 cm 3.
Water vapor is present in the gas. Since H2 is created during the reaction and water vapor is irrelevant when determining the equivalent weight, we only need the dry gas. As a result, the volume of H2 gas can be calculated using the ideal gas law.
P 1 = 750 mm Hg, 14 mm Hg, 736 mm Hg V = 50 cm 3 T = 17 0 C = 290 K
P = 760mmHg, V =?cm3, T = 0 0 and C = 273K.
The Gas equation follows.
P 1V 1/ T1 = P 2V 2/ T2
290/73650 = 273/760V 2
= 290760V 2
= 7365027V 2 = 45.58 cm
As a result, the dry gas volume at STP is 45.58 cm 3.
Learn more about Volume here-
https://brainly.com/question/1578538
#SPJ1
a sample of a gas at room temperature occupies a volume of 38.0 ll at a pressure of 782 torrtorr . if the pressure changes to 3910 torrtorr , with no change in the temperature or moles of gas, what is the new volume, v2v2v 2 ?
Answers
The new volume of sample gas at room temperature with no change in temperature or moles of gas is 7.6 liters
Calculation→
The new volume is calculated using Boyle's law formula
That is P₁V₁=P₂V₂
where,
p₁ = 782 torr
V₁ = 38.0 l
P₂ = 3910 torr
V₂= New volume
make V₂ the subject of the formula by diving both side of equation bP₂
V₂ = P₁V₁/P₂
V₂ is therefore = {(38.0 l × 782 torr) 3910 torr} = 7.6 L
Learn more about volume here:-
https://brainly.com/question/11711850
#SPJ4