Calculate the mass of a sphere of gold with a radius of 11.3 cm. (The volume of a sphere with a radius r is V = (4/3)πr3; the density of gold is 19.3 g/cm3.) Express the solution in grams and in scientific notation.
Answers
The mass of a sphere with a radius of 11.3 cm can be calculated using the equation M = V × ρ, where V is the volume of the sphere and ρ is the density of the material. The volume of a sphere with a radius r is V = (4/3)πr3 and the density of gold is 19.3 g/cm3, so we can calculate the mass of the gold sphere as:
M = (4/3)πr3 × 19.3 g/cm3 = (4/3) × 3.14 × 11.33 × 19.3 g/cm3
M = 8,683.29 g = 8.7 × 103 g (in scientific notation)
Read more about the topic of density:
https://brainly.com/question/1354972
#SPJ11
Related Questions
Element 1 2 3 4 5 Electronegativity 3.04 1.88 2.04 2.20 3.98 Which element would be most likely to identify as a metal
Answers
The element with the highest electronegativity value in the given list is 3.98, which corresponds to Element 5. Therefore, Element 5 would be the most likely to identify as a metal.
To know more about electronegativity and its relationship to metal identification, refer here:
Electronegativity is a measure of an element's ability to attract electrons in a chemical bond. Metals typically have lower electronegativity values compared to nonmetals. They tend to lose electrons easily to form positive ions and exhibit metallic properties such as conductivity, malleability, and luster.
In the given list, Element 5 has the highest electronegativity value of 3.98. This value is significantly higher than the other elements in the list. The large electronegativity suggests that Element 5 has a strong ability to attract electrons, which is characteristic of nonmetals rather than metals. Therefore, the remaining elements in the list (Elements 1, 2, 3, and 4) would be more likely to identify as metals.
In summary, Element 5, with an electronegativity value of 3.98, would be the least likely to identify as a metal among the given elements.
To know more about electronegativity, refer here:
https://brainly.com/question/3393418#
#SPJ11
What is the systematic name of HNO2?
Answers
Nitrous acid is the chemical name for HNO₂ and it is its systematic name.
Nitric acid has the chemical formula HNO₃. Compared to nitrous acid, it is more potent and stable. This is due to the fact that its conjugate base is more stable than that of nitrous acid. H₂N₂O₂ is the chemical formula for hyponitrous acid. HNO₂ is a wobbly, unstable, and mildly acidic substance. It is sometimes referred to as a nitrogen oxoacid. Its molecular weight is 47 g/mol. HNO₂ has a boiling point of 158°C.
Nitrous acid is commonly produced by acidifying nitrite salt with mineral acid. As a result, nitrous acid is the chemical term or the systematic name for HNO₂.
To know more about nitrous acid, visit,
https://brainly.com/question/28166496
#SPJ4
what should you do if you are wearing a tie in lab day? what do you think your teacher will suggest other than removing the tie?
Answers
Answer: Probably why are you wearing it and why do you have it on
Explanation:
Answer:
remove it, tuck it under ur shirt
Explanation:
Plz answer this question

Answers
Answer: i dont know why the metal was chosen, but the original metal used on the statue of liberty was bronze :)
Explanation:
Which is the best argument for why CO2 represents a covalent bond?
a
The difference in electronegativity of the two elements is greater than 1.7.
b
The difference in electronegativity of the two elements is less than 1.7.
c
The difference in electronegativity of the two elements is greater than 1.7 but less than 3.0.
d
The difference in electronegativity of the two elements is zero.
Answers
Answer:
B.) The difference in electronegativity of the two elements is less than 1.7.
Explanation:
A difference of less than 2 between atoms leads to covalent bond formation. Electronegativity is the ability of an atom to draw bonding electrons when it is bonded.
We know that in covalent bonding, atoms "share" elections. We also know that in ionic bonding, atoms "take" electrons. If the electronegativity was too great then atoms would no longer share electrons, one would just draw the electron to it. The ideal values to covalently bond, in this case, would be below two.
Use the chart to determine which pair of atoms has the greatest difference in electronegativity.
A. Ca – Cl
B. H – Cl
C. Fe – Cl
D. P – Cl
Answers
The electronegativity for chlorine is 3.2. Now, we must look for the electronegativity that has the greatest difference from chlorine.
Calcium (Ca) - 1.0
Hydrogen (H) - 2.2
Iron (Fe) - 1.8
Phosphorus (P) - 2.2
As you can see, the element with the lowest electronegativity level is Calcium, therefore the greatest difference in electronegativity would be present between Calcium and Chlorine.
How does the idea of quantized energy apply to Bohr’s atomic model?

Answers
Answer:the electrons can possess only certain discrete energy values; values between those quantized values are not permitted.
Explanation:Both involve a relatively heavy nucleus with electrons moving around it, although strictly speaking, the Bohr model works only for one-electron atoms or ions.
How many molecules in 35 g of CO2
Answers
Answer:
35/44* 6.022*10^24
Please mark as brainliest
Explanation:

there is an ice cream shop 6 blocks north of your hotel. how many minutes will it take to walk there and back
Answers
Answer:
This question is incomplete
Explanation:
This question is incomplete.
However, when all the required data are available, you can use the formula/steps below
Average speed/velocity (m/s) = distance (in metres) ÷ time (in seconds)
Time (in secs) = distance ÷ average speed/velocity
Kindly note that "blocks" is not a standard unit for distance measurement in science, hence the distance (since its a walking distance) must be in metres (or converted to metres if not in metres).
The distance in the formula is the distance from the hotel to the ice cream shop while the average speed is the distance covered per time as s/he walks to the ice cream shop and back. Hence, the answer gotten from the formula above will have to be multiplied by 2 (in order to get the time taken to walk to the ice cream shop and back) because the formula will only provide answer to one trip (time taken to walk to the shop).
After the multiplication mentioned above, the time in seconds should be converted to minutes by dividing the answer in seconds by 60
What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
A 510 kg sailboat is being pulled with a rope by a truck with a force of 965 N causing the boat to accelerate at 1.8 m/s2 to the left.
Answers
There's a small discrepancy between the applied force (965 N) and the calculated force (918 N), which might be due to rounding or measurement errors.
To calculate the force required to accelerate the sailboat, we can use the formula F = m*a, where F is the force, m is the mass, and a is the acceleration.
So, F = (510 kg)*(1.8 m/s^2) = 918 N.
However, in this scenario, the truck is pulling the sailboat with a force of 965 N, which is greater than the force required to accelerate the boat. This means that the boat will not only accelerate to the left, but it will also experience a net force to the right.
To find the magnitude of this net force, we can use the formula F_net = F_applied - F_friction, where F_applied is the force applied by the truck and F_friction is the force of friction acting in the opposite direction.
Since the sailboat is being pulled on a flat surface, we can assume that the force of friction is negligible. Therefore, F_net = F_applied = 965 N.
Now, to find the time it takes for the sailboat to reach a velocity of 150 m/s to the left, we can use the formula v = a*t, where v is the velocity, a is the acceleration, and t is the time.
Rearranging the formula, we get t = v/a. Substituting in the given values, we get t = (150 m/s) / (1.8 m/s^2) = 83.3 seconds.
So, the long answer to your question is that the sailboat will experience a net force of 965 N to the right, and it will take 83.3 seconds to reach a velocity of 150 m/s to the left.
a 510 kg sailboat is being pulled by a truck with a force of 965 N, which results in an acceleration of 1.8 m/s² to the left. Using Newton's second law (F = m * a), we can check if the force applied by the truck is consistent with the sailboat's mass and acceleration:
F = m * a
965 N = 510 kg * 1.8 m/s²
Solving for the force, we get:
965 N = 918 N
To know more about acceleration visit:-
https://brainly.com/question/12550364
#SPJ11
What is the density of a material if a sample with a mass of 45.82 g takes up a volume of 8.75 mL?
Answers
So here density = 45.82g/8.75ml
= 5.23g/ml
A 3. 8
g sample of sodium hydrogen carbonate is added to a solution of acetic acid weighing 10. 5
g. The two substances react, releasing carbon dioxide gas to the atmosphere. After the reaction, the contents of the reaction vessel weigh 11. 7
g. What is the mass of carbon dioxide released during the reaction?
Answers
The mass of carbon dioxide released during the reaction is 2.6 grams.
To determine the mass of carbon dioxide released during the reaction between sodium hydrogen carbonate (NaHCO3) and acetic acid (CH3COOH), we need to calculate the difference in mass before and after the reaction.
Before the reaction:
Mass of NaHCO3 = 3.8 g
Mass of acetic acid = 10.5 g
Total mass before the reaction = Mass of NaHCO3 + Mass of acetic acid = 3.8 g + 10.5 g = 14.3 g
After the reaction:
Mass of the contents of the reaction vessel = 11.7 g
To find the mass of carbon dioxide released, we calculate the difference in mass:
Mass of carbon dioxide released = Total mass before the reaction - Mass of the contents of the reaction vessel
= 14.3 g - 11.7 g
= 2.6 g
Therefore, the mass of carbon dioxide released during the reaction is 2.6 grams.
learn more about carbon dioxide here
https://brainly.com/question/3049557
#SPJ11
A molecule, that is sp3d2 hybridized and has a molecular geometry of square pyramidal, has ________ bonding groups and ________ lone pairs around its central atom a molecule, that is hybridized and has a molecular geometry of square pyramidal, has ________ bonding groups and ________ lone pairs around its central atom
Answers
A molecule that is sp³d² hybridized and has a molecular geometry of square pyramidal has 5 bonding groups and 1 lone pair around its central atom.
A molecule that is hybridized and has a molecular geometry of square pyramidal has 6 bonding groups and 0 lone pairs around its central atom. The hybrid orbitals are directed towards the vertices of a square pyramid.
In the sp³d² hybridization, the central atom has a total of 6 electron domains, consisting of 5 bonding groups (each representing a bond with another atom) and 1 lone pair (representing a pair of non-bonding electrons).
The arrangement of these electron domains results in a molecular geometry of square pyramidal, where the bonding groups occupy the corners of a square base and the lone pair is located above the center of the square base, giving it a pyramidal shape.
To know more about the molecular geometry refer here :
https://brainly.com/question/31984103#
#SPJ11
a substance is analyzed to have a percent composition of 74.186% sodium and 25.814% fluorine. calculate the empirical formula
Answers
Answer:
Na₂₆F₁₁
Explanation:
We find the moles of the substance assuming 100 g of the substance is present. Why do we take 100 g? Because then the percent of sodium/fluorine, would be the g of sodium/fluorine respectively:
74.186 g Sodium | 1 mol Sodium/23 g => 3.2255 mol Na
25.814 g Fluorine | 1 mol Fluorine/19 g => 1.3586 mol F
Divide each by smallest number of moles:
3.2255/1.3586 = 2.37
1.3586/1.3586 = 1
Multiply by common number to get a smallest whole number:
2.37*11 = 26,
1*11 = 11
The empirical formula is Na₂₆F₁₁
color in a unit cell of this two-dimensional lattice:
Answers
The color in a unit cell of a two-dimensional lattice is determined by the arrangement of atoms within the lattice structure. A unit cell is the smallest repeating unit within the lattice, and it contains all of the atoms within the lattice.
The atoms are arranged in a regular pattern, which can be described as a crystal lattice.The color in a unit cell is determined by the type of bonds that exist between the atoms in the lattice. Different types of bonds will produce different colors. For example, ionic bonds are strong and result in a bright, vivid color, while covalent bonds are weaker and produce a more muted color. Metals, on the other hand, have metallic bonds, which produce a metallic sheen.
The color of a unit cell can also be affected by the properties of the atoms themselves. For example, if the atoms have a large difference in electronegativity, the unit cell may appear to have a strong color contrast. Additionally, the color can vary depending on the angle at which it is viewed.In summary, the color in a unit cell of a two-dimensional lattice is determined by the type of bonds that exist between the atoms, as well as the properties of the atoms themselves. These factors will determine the overall appearance of the unit cell.
Learn more about unit cell at :https://brainly.com/question/13110055
#SPJ4
What is the correct equilibrium constant expression for the following reaction?
2NCl3(g) Û N2g) + 3Cl2g
Answers
Answer:
K = [N2] [Cl2]³ / [NCl3]²
Explanation:
The equilibrium expression, K, of a reaction:
aA + bB ⇄ cC + dD
Is defined as the ratio between the multiplication of concentrations of products powered to its reaction coefficient and the multiplication of concentrations of reactants powered to its reaction coefficient as follows:
K = [C]^c[D]^d / [A]^a[B]^b
Now, for the reaciton:
2NCl3 ⇄ N2(g) + 3Cl2(g)
K is:
K = [N2] [Cl2]³ / [NCl3]²
Iron is a
homogenous mixture
O heterogeneous mixture
O element
compound
Answers
Answer:
element
Explanation:
the solubility of SrCO3 in water at 25C is measured to be 0.0045 g/L. Use this information to calculate K_sp for SrCO3. Round your answer to 2 significant digits.
Answers
\(K_{sp\), solubility product constant for SrCO₃ is approximately 9.3 x 10⁻¹⁰.
To find the solubility product constant (\(K_{sp\)) for SrCO₃, we'll first need to write the balanced chemical equation and determine the molar solubility.
Balanced chemical equation: SrCO₃(s) ⇌ Sr²⁺(aq) + CO₃²⁻(aq)
From the given solubility of 0.0045 g/L, we can calculate the molar solubility. The molar mass of SrCO₃ is approximately 147.63 g/mol.
Molar solubility = (0.0045 g/L) / (147.63 g/mol) ≈ 3.05 x 10⁻⁵ mol/L
Now, let's express the equilibrium concentrations in terms of x, where x is the molar solubility of SrCO₃:
[Sr²⁺] = x = 3.05 x 10⁻⁵ mol/L
[CO₃²⁻] = x = 3.05 x 10⁻⁵ mol/L
\(K_{sp\) is the product of the equilibrium concentrations of the ions:
\(K_{sp\) = [Sr²⁺][CO₃²⁻] = (3.05 x 10⁻⁵)(3.05 x 10⁻⁵) ≈ 9.30 x 10⁻¹⁰
Rounded to two significant digits, \(K_{sp\) for SrCO₃ at 25°C is approximately 9.3 x 10⁻¹⁰.
To know more about solubility product constant, refer to the link below:
brainly.com/question/31605015#
#SPJ11
Which best describes MgO?
1)This is an ionic compound; it is named
magnesium monoxide.
2)This is a covalent compound; it is named
magnesium monoxide.
3)This is an ionic compound; it is named
magnesium oxide.
4)This is a covalent compound; it is named
magnesium oxide.
Answers
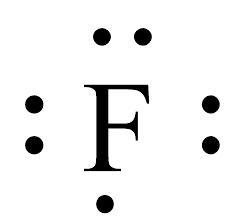
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
What characteristic of light determines what color it appears?
Answers
Answer:
Light is made up of wavelengths of light, and each wavelength is a particular colour. The colour we see is a result of which wavelengths are reflected back to our eyes. The visible spectrum showing the wavelengths of each of the component colours. The spectrum ranges from dark red at 700 nm to violet at 400 nm.The 'colour' of an object is the wavelengths of light that it reflects. This is determined by the arrangement of electrons in the atoms of that substance that will absorb and re-emit photons of particular energies according to complicated quantum laws.The reason that different waves of light appear to be different colors of light is because the color of a light wave depends on its wavelength. For example, the wavelength of blue light is about 450 nanometers, while the wavelength of red light is about 700 nanometers.
Explanation:
Which atom (magnesium or chlorine) is larger? _______________________(you should also be prepared to answer the question if asked for the smaller atom)3a. Explain why the atom is larger. Include the following terms in your answer: protons, electrons, shells or layers, columbic attractions
Answers
Answer:
The magnesium atom is larger.
Explanation:
The magnesium atom is larger because it is on the left side of the Periodic Table (period 3 and group 2) where the atomic radius is larger.
Atomic radius is the distance from the center of the nucleus of an atom to the most external electron shell.
The greater the columbic attractions, the closer the protons in the nucleus are to the electrons in the outer layers, making the size of the atom smaller.
Calculate the average acceleration of a car with a starting velocity of 0 m/s that accelerates to 9
m/s in 3 seconds.
Answers
Answer:
3 m/s
Explanation:
I found the unit rate which is 9/3 = 3
A(n) _____ is a hydrocarbon in which one or more hydrogen atoms is replaced by a -COOH and a -NH2 group. aldehyde carboxylic acid alcohol amino acid
Answers
Answer:
Amino Acid
Explanation:
It's part of the Amino group, which are substituted hydrocarbons.
When NH₂ and COOH replaces hydrogens on the same carbon atom in a molecule, an Amino acid is formed.
What group is a COOH?
COOH : a Carboxylic Acid group!
Are the following chemical equations reversible or irreversible?
2H2O ←→ H3O+ + OH-
HA + H2O ←→ A- + H3O+
HA + H2O → A- + H3O+
MOH → M+ + OH-
Answers
The first two chemical equations are reversible while the other two are irreversible.
What are chemical equations?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equations,here:
https://brainly.com/question/19626681
#SPJ1
Can you show me the answer and explain?

Answers
Answer:
C
Explanation:
looking at a periodic table X is fluorine and Y is potassium
Fluorine is in group 7 and forms a 1- charge (which gains electrons) and potassium is in group 1 and forms a 1+ charge (which loses electrons)
Fluorine (X) has an electronic structure of 2,7 and needs to gain an electron from Potassium (Y) to have a full outer shell and potassium has an electronic structure of 2,8,8,1 so needs to lose an electron to have a full outer shell as well. This means that the electron that potassium (Y) has lost is given away to fluorine (X), so both elements become stable.
This is known as ionic bonding where metals (like potassium) lose electrons and non-metals (like fluorine) gain electrons to become more stable, forming ions
Any further clarification let me know
What minerals are formed with covalent bonds?
What common mineral forms from solution by precipitation?
Answers
Minerals formed with covalent bonds include diamond and graphite. Diamond has a three-dimensional network structure, while graphite has layered structures. Halite is a common mineral that forms from solution by precipitation when saltwater evaporates, resulting in the formation of halite crystals.
Covalent bonds are formed when two atoms share electrons, resulting in a strong bond. Several minerals are formed with covalent bonds, including diamond and graphite. Diamond is composed entirely of carbon atoms bonded together through covalent bonds, creating a three-dimensional network.
This gives diamond its hardness and brilliance. On the other hand, graphite consists of layers of carbon atoms arranged in a hexagonal pattern, with covalent bonds within each layer but weak bonds between the layers. Graphite is soft and has a slippery texture due to the weak interlayer bonds.
One common mineral that forms from solution by precipitation is halite, also known as rock salt. Halite forms when saltwater evaporates, leaving behind the mineral. This process occurs in areas with a high concentration of saltwater, such as salt flats or saltwater lakes. As the water evaporates, the dissolved salt ions come together and form solid halite crystals. These crystals can be further processed to produce table salt that we use in cooking.
To know more about mineral visit:
https://brainly.com/question/29970865
#SPJ11
Kayla learned that when you play with the Soccket for 30 minutes it gives you 3 hours of light. She thinks that rolling the ball slowly with her baby brother for 30 minutes will give her the same amount of energy as kicking it in a game of soccer for 30 minutes. Do you agree or disagree? State your claim and use evidence from what you've learned about speed and energy to explain your answer.
Answers
Answer:
disagree it wont be the same momentum
Explanation:
cuh
Consider the following half-reaction balanced for an acidic solution: 2H2O + SeO2 → SeO42- + 4H+ + 2e-. What is the balanced half-reaction for a basic solution?
Answers
Answer
\(SeO_2+4OH^-\rightarrow SeO^{2-}_4+2H_2O+2e^-\)Explanation
The given balanced half-reaction for an acidic solution:
\(2H_2O+SeO_2\rightarrow SeO^{2-}_4+4H^++2e^-\)What to find:
Tha balanced half-reaction for a basic solution.
Step-by-step-solution:
To balance the half-reaction for a basic solution;
1. Add OH⁻ ions to BOTH SIDES to neutralize any H⁺
\(2H_2O+SeO_2+4OH^-\rightarrow SeO^{2-}_4+4H^++4OH^-+2e^-\)2. Combine H+ and OH- to make H2O.
\(2H_2O+SeO_2+4OH^-\rightarrow SeO^{2-}_4+4H_2O+2e^-\)3. Simplify by canceling out excess H2O
\(SeO_2+4OH^-\rightarrow SeO^{2-}_4+2H_2O+2e^-\)4. Balance the charges by adding e-
\(SeO_2+4OH^-\rightarrow SeO^{2-}_4+2H_2O+2e^-\)