Calculate the molarity (moles/L) of acetic acid in vinegar: Use the molar mass of acetic acid to convert your molarity value above to grams of acetic acid per mL Take this number times [00 to get & percent acetic acid in vinegar: (The result should be close to 5%.)
Answers
Calculating the molarity of acetic acid in vinegar:
Molarity (M) = (number of moles of solute) / (volume of solution in liters)
What is molar mass?The molar mass is the same as mass number if it is only one element with no subscripts.
the mass of acetic acid in the vinegar will be determined first:
Mass = volume (L) × density (g/mL)
Mass = 1 L × 1.05 g/mL
Mass = 1.05 g/L
Then, the moles of acetic acid can be calculated using the molar mass of acetic acid:
Moles = mass (g) / molar mass
Moles = 1.05 g / 60.05 g/mol
Moles = 0.01748 mol
Acetic acid molarity = 0.01748 mol / 1 L
= 0.01748 M
Calculating the percentage of acetic acid in vinegar:
% acetic acid = (mass of acetic acid/volume of vinegar) × 100%
= (1.05 g / 100 mL) × 100%
= 1.05%
Therefore, the result of the calculation will be close to 1.05%, not 5%.
To know more about molarity:
https://brainly.com/question/19517011
#SPJ11
Related Questions
A sphere of radius 0.457 m, temperature 32.2 ∘
C, and emissivity 0.924 is located in an environment of temperature 82.9 ∘
C. At what rate does the sphere (a) emit and (b) absorb thermal radiation? (c) What is the sphere's net rate of energy exchange? (a) Number (b) Number Units Units
Answers
a) The sphere emits thermal radiation at a rate of 139.75 Watts.
b) The sphere absorbs thermal radiation at a rate of 37.66 Watts.
c) The sphere's net rate of energy exchange is 102.09 Watts.
What are the rates of thermal radiation emission, absorption, and net energy exchange for the sphere?To calculate the rates of thermal radiation emission and absorption, we can use the Stefan-Boltzmann law, which states that the rate of thermal radiation emitted or absorbed by an object is proportional to its surface area, temperature, and the Stefan-Boltzmann constant.
a) The rate of thermal radiation emitted by the sphere can be calculated using the formula:
Emitting Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(temperature^4 - environment\ temperature^4\))
Plugging in the given values:
Emitting Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((32.2 + 273.15)^4 - (82.9 + 273.15)^4)\)
Emitting Rate ≈ 139.75 Watts
b) The rate of thermal radiation absorbed by the sphere can be calculated in a similar way but using the environment temperature as the object's temperature:
Absorbing Rate = emissivity * surface area * Stefan-Boltzmann constant * (\(environment\ temperature^4 - temperature^4\))
Plugging in the given values:
Absorbing Rate = \(0.924 * (4\pi * (0.457)^2) * 5.67 \times 10^{-8} * ((82.9 + 273.15)^4 - (32.2 + 273.15)^4)\)
Absorbing Rate ≈ 37.66 Watts
c) The net rate of energy exchange is the difference between the emitting rate and the absorbing rate:
Net Rate = Emitting Rate - Absorbing Rate
Net Rate = 139.75 Watts - 37.66 Watts
Net Rate ≈ 102.09 Watts
Therefore, the sphere emits thermal radiation at a rate of 139.75 Watts, absorbs thermal radiation at a rate of 37.66 Watts, and has a net rate of energy exchange of 102.09 Watts.
Note: The units for all the rates are Watts.
Learn more about thermal radiation emission
brainly.com/question/28517392
#SPJ11
What is carbocylic compound??
Answers
Carbocyclic compounds are compounds that have form a carbon atom rings.
What is Carboxylic CompoundsCarbocyclic compounds e.g. aromatic or non-aromatic cyclic carbon compounds are those compound that have a carbon ring attached or enclosed to them. A good example of organic compound freely occurring in nature are of this form. The main parent of these compounds are either benzene of cyclohexane or cyclohexene.
Learn more on carbocyclic compounds here;
https://brainly.com/question/9165411
3.00 moles Ce2(CO3)3 to grams
Answers
The mass (in grams) present in 3.00 moles of Ce₂(CO₃)₃ is 1380.696 grams
How do I determine the mass of 3.00 moles of Ce₂(CO₃)₃?We'll begin by obtaining the molar mass of Ce₂(CO₃)₃. This can be obtained as follow:
Molar mass of Ce = 140.116 g/mol Molar mass of C = 12 g/molMolar mass of O = 16 g/molMolar mass of Ce₂(CO₃)₃ = ?Molar mass of Ce₂(CO₃)₃ = molar mass of individual elements
Molar mass of Ce₂(CO₃)₃ = (140.116 × 2) + 3[12 + (3 × 16)]
Molar mass of Ce₂(CO₃)₃ = 460.232 g/mol
Finally, we can obtain the mass of 3.00 moles of Ce₂(CO₃)₃. Details below:
Mole of Ce₂(CO₃)₃ = 3.00 moles Molar mass of Ce₂(CO₃)₃ = 460.232 g/mol Mass of Ce₂(CO₃)₃ =?Mole = mass / molar mass
Cross multiply
Mass = Mole × molar mass
Mass of Ce₂(CO₃)₃ = 3 × 460.232
Mass of Ce₂(CO₃)₃ = 1380.696 grams
Thus, the mass of Ce₂(CO₃)₃ is 1380.696 grams
Learn more about mass:
https://brainly.com/question/6613610
#SPJ1
PLEASE I WILL BRAINLIST YOU
1. A student predicted the reaction between hydrogen and oxygen gas would yield 125 mL of water. However, after measuring the water formed in the reaction, the student determined that only 110 mL of water was actually formed. Determine the percent yield for this reaction.
A. 75%
B. 88%
C. 100%
D. 113%
HINT: 96 yield= (actual yield/ expected yield ) X 100%
Answers
Answer:
B. 88%
Explanation:
percent yield is the actual yield divided by the theoretical (predicted) yield, then multiplied by 100 to get a percentage.
110/125 = 0.88
0.88 x 100 = 88%
In chemistry, the term yield also known as the reaction yield represents the measure of the quantity of the moles of a product formed in the reaction to the reactant used. The correct option is B.
What is percent yield?The amount of the product actually made compared with the maximum calculated yield is defined as the percent yield. It can also be known as the ratio of the actual yield to the theoretical yield.
The ideal quantity of the product is called the theoretical yield and it is obtained by working a stoichiometry problem. The actual amount of the product formed gives us the actual yield.
The equation of the percent yield is given as:
%yield = Actual yield / Theoretical yield × 100
110 / 125 × 100 = 0.88 × 100 = 88%
Therefore the percent yield of the reaction is 88%.
Thus the correct option is B.
To know more about percent yield, visit;
https://brainly.com/question/29714892
#SPJ2
A student is working hard on a chemistry lab experiment that uses a strong acid. Halfway through the lab, the student gets hungry and starts eating a bag of chips. When the student licks their fingers, they start to have a severe reaction. summary
Answers
How many sulfur (S) atoms in copper (II) sulfate pentahydrates?
Answers
Answer: In copper (II) sulfate pentahydrate, only 1 sulfur atom is present.
Explanation:
The chemical formula of the copper (II) sulfate pentahydrate is \(CuSO_4.5H_2O\)
In the given chemical formula:
Number of copper atoms = 1
Number of sulfur atoms = 1
Number of oxygen atoms = 9
Number of hydrogen atoms = 10
Hence, in copper (II) sulfate pentahydrate, only 1 sulfur atom is present.
A woman is deciding between three alarm systems to install in her home. Each alarm system produces a wave with a different wavelength and decibel level, as shown in the following table.
If the woman wants the alarm with the lowest pitch, which alarm should she choose? Explain your answer.
Based on the data in the table, which alarm produces sound waves with the greatest amplitude? Explain your answer.
Write 3–4 sentences identifying which alarm has the lowest pitch and explain why you chose that alarm?
Write 3–4 sentences identifying the alarm that produces a sound with the greatest amplitude and explain why you chose that alarm?
Answers
Answer:
Based on the data in the table, which alarm produces sound waves with the greatest amplitude? Explain your answer.
this bit says based on the table but there is no table.
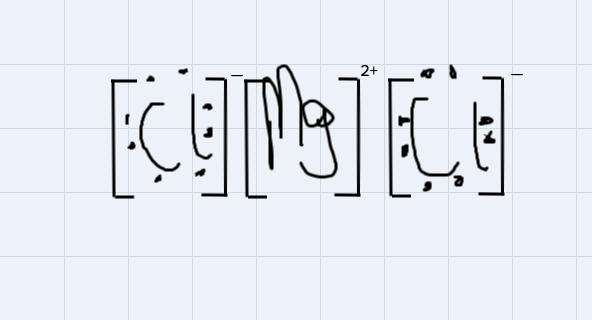
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
when a mg2 ion becomes a mg atom, the radius increases because the mg2 ion
Answers
The radius of the Mg atom increases because the Mg²⁺ has gained 2 extra electrons and a new shell has also been added.
Magnesium atom and ionGeneral, magnesium atom, Mg has 3 electron shells while magnesium ion, Mg²⁺ has 2 electron shells.
Since Mg is a metal, its atom is bigger that its ion, Mg²⁺.
Thus, to move from Mg²⁺ to Mg, the ion will need 2 extra electrons as shown below:
Mg²⁺ + 2e —> Mg
Since the Mg atom is bigger, it also means that the radius of the Mg is bigger than the radius of the Mg²⁺.
See attached photo for further details
Learn more about atoms and ions:
https://brainly.com/question/491497

A large scuba tank with a volume of 18 L is rated for a pressure of 220 bar. The tank is filled at 20 °C and contains enough air to supply 1860 L of air to a diver at a pressure of 2.37 atm (a depth of 45 feet). Was the tank filled to capacity at 20 °C?
Answers
Answer:
Yes, at 20 °C the tank was filled to full capacity
Explanation:
Given;
let initial pressure, P₁ = 2.37 atm
let initial volume, V₁ = 1860 L
let final pressure, P₂ = 220 bar = 217.123 atm
let final volume, V₂ = ?
Subject the air volume (1860 L ) at 2.37 atm to the tank rating (220 bar)
Ideal law is given by;
PV = nRT
At a fixed mole and gas temperature, the equation becomes;
P₁V₁ = P₂V₂
V₂ = (P₁V₁) / P₂
V₂ = (2.37 x 1860) / 217.123
V₂ = 20.3 L
After subjecting the air volume to tank rating, the volume of the air at 220 bar is 20.3 L which is greater than tank volume (18 L).
The extra 2.3 L can be assumed to be compressed gas volume due to the given (lower) temperature.
Thus, at 20 °C the tank was filled to full capacity.
If 3.00 mL of 0.0250 M CuSO4 is diluted to 25.0 mL with pure water, what is the molarity of copper(II) sulfate in the diluted solution
Answers
Answer:
0.00268 M
Explanation:
To find the new molarity, you need to (1) find the moles of CuSO₄ (via the molarity equation using the beginning molarity and volume) and then (2) find the new molarity (using the moles and combined volume). Your final answer should have 3 sig figs to match the given values.
Step 1:
3.00 mL / 1,000 = 0.00300 L
Molarity = moles / volume (L)
0.0250 M = moles / 0.00300 L
(0.0250 M) x (0.00300 L) = moles
7.50 x 10⁻⁵ = moles
Step 2:
25.0 mL / 1,000 = 0.0250 L
0.0250 L + 0.00300 L = 0.0280 L
Molarity = moles / volume (L)
Molarity = (7.50 x 10⁻⁵ moles) / (0.0280 L)
Molarity = 0.00268 M
cyclopentane c5h10 is an alkane with a ring structure, that reacts with chlorine, cl2, to produce c2h9cl and hcl. following is a representation of a proposed mechanism for the reaction. Cl2-->2CL (Slow) Cl+C5H10-->HCl+C5H9 (Fast) C5H9+Cl-->C5H9Cl (Fast). (a0 Write the overall equation for the reaction. (b)Write a rate for the reaction that is consistent with the mechanism. Justify your answer (c) A student claims that cl2 is a catalyst in the reaction. Explain why the students claim is false.
Answers
A. We are aware that the reaction's rate depends on the slowest, or rate-determining, step in the overall reaction mechanism. The first step of the reaction is the one that moves the most slowly in the example reaction mechanism. As a result, just one step (Step 1) would have an impact on the reaction's rate.
As a result, the rate law for the mechanism described is given by –
Step 1. Cl2 2Cl (slow) ---------k1
Step 2. Cl + C5H10HCl + C5H9 (fast) ---------k2
Step 2. C5H9 + Cl C5H9Cl (fast) ---------k3
Step 1. is the slowest step. So, the reaction rate will depend upon on the rate of Step 1 only.
Hence, the rate law for the reaction is given by –
Rate = k1 [Cl2]
B. A species that is created and then consumed in situation is referred to as an intermediate of a reaction mechanism; hence, reaction intermediates never appear in the final rate law or in the actual reaction itself. The overall reaction in this reaction will show up, and likewise will be included in the rate law, as demonstrated above.
To learn more about Rate Law Reaction:
https://brainly.com/question/8139015
#SPJ1
The buffer ph most effective at allowing amphoteric proteins to migrate toward the cathode in an electrophoretic system would be:____.
Answers
The buffer ph most effective at allowing amphoteric proteins to migrate toward the cathode in an electrophoretic system would be 4.5
What are buffers in pH?A buffer is an aqueous solution that can withstand considerable pH changes when modest amounts of acid or alkali are added.Each buffer has a specific "capacity," which is the amount of strong acid or base needed to raise the pH of one liter of solution by one pH unit.The pH of an acetic acid and sodium acetate solution in water is equivalent at 4.74.These solutions have a pH that is under seven. Solutions are made up of a weak acid.A mixture of sodium acetate and acetic acid (pH = 4.75) serves as an illustration of an acidic buffer solution.Learn more about buffer ph here:
https://brainly.com/question/13076037
#SPJ4
The buffer ph most effective at allowing amphoteric proteins to migrate toward the cathode in an electrophoretic system would be 4.5
What are buffers in pH?A buffer is an aqueous solution that can withstand considerable pH changes when modest amounts of acid or alkali are added.Each buffer has a specific "capacity," which is the amount of strong acid or base needed to raise the pH of one liter of solution by one pH unit.The pH of an acetic acid and sodium acetate solution in water is equivalent at 4.74.These solutions have a pH that is under seven.Solutions are made up of a weak acid.A mixture of sodium acetate and acetic acid (pH = 4.75) serves as an illustration of an acidic buffer solution.Learn more about buffer ph here:
brainly.com/question/13076037
#SPJ4
Which of the following biosensor recognition elements are based on an organism's immune response (multiple correct answers possible)? a. aptamers b. antibodies c. carbohydrates d. peptides
Answers
The biosensor recognition elements that are based on an organism's immune response include b. antibodies and d. peptides. Antibodies are proteins produced by the immune system in response to foreign substances, while peptides can also participate in immune responses by acting as signaling molecules or antimicrobial agents.
Both antibodies and peptides are biosensor recognition elements that are based on an organism's immune response. Antibodies are proteins produced by the immune system in response to specific antigens, and they bind to these antigens with high specificity and affinity. Peptides are short chains of amino acids that can be recognized by T cells, another component of the immune system. Aptamers, on the other hand, are synthetic molecules that can bind to specific targets with high affinity and specificity, but they are not based on immune recognition. Carbohydrates also do not typically play a role in biosensor recognition based on immune response. So, the correct answers to this question are b. antibodies and d. peptides.
To know more about Antibodies visit:
https://brainly.com/question/27931383
#SPJ11
9. A circuit contains a 1200 12, a 220 92, and a 3300 12 resistor connected in parallel. The circuit has a total
current flow of 0. 25A. How much current flows through each resistor?
Answers
To determine the current flowing through each resistor in a parallel circuit, we can use Ohm's Law and the concept of equivalent resistance.
In a parallel circuit, the voltage across each resistor is the same, while the current is divided among the resistors. We can calculate the current flowing through each resistor using the following formula:
I = V/R
Where:
I is the current flowing through the resistor,
V is the voltage across the resistor, and
R is the resistance of the resistor.
Let's calculate the current flowing through each resistor:
For the 1200 Ω resistor:
I₁ = V/R = 12 V / 1200 Ω = 0.01 A or 10 mA
For the 220 Ω resistor:
I₂ = V/R = 12 V / 220 Ω ≈ 0.055 A or 55 mA
For the 3300 Ω resistor:
I₃ = V/R = 12 V / 3300 Ω ≈ 0.0036 A or 3.6 mA
Therefore, the current flowing through the 1200 Ω resistor is approximately 10 mA, the current flowing through the 220 Ω resistor is approximately 55 mA, and the current flowing through the 3300 Ω resistor is approximately 3.6 mA.
Learn more about equivalent resistance here:
https://brainly.com/question/31788815
#SPJ11
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
unless otherwise instructed, you may use the periodic table in the chemistry: problems and solutions book for this question. what is the branch of chemistry that examines numerical relationships in chemical reactions?
Answers
The branch of chemistry is Stoichiometry
Chemistry's study of numerical relationships in chemical reactions is known as stoichiometry. The Law of Conservation of Mass serves as its foundation. Although the elements that reactants and products are associated with change frequently during a reaction, all chemical reactions contain the same elements as reactants and products.
Any chemical equation must be balanced, and this is done by comparing the number of atoms utilized as reactants and products. In essence, it denotes how the reactant and product are related. There is no qualitative analysis involved; merely a quantitative one. n To determine the amount of moles used in the reactant and product of the particles, stoichiometry is the study of chemical reactions.
Read more about Stoichiometry on:
https://brainly.com/question/20802034
#SPJ4
a sample of a material has a mass of 48 grams and a volume of 6 cubic centimeters. what is the density of this sample/
Answers
Density is defined as the mass per unit volume of a substance. The formula for density is: density = mass / volume. Therefore, the density of the sample is 8 g/cm³.
Given that the mass of the material is 48 grams and the volume is 6 cubic centimeters, we can use the formula to calculate the density:
density = 48 g / 6 cm³
Simplifying the expression, we get:
density = 8 g/cm³
Therefore, the density of the sample is 8 g/cm³.
Density is a physical property of matter that describes how much mass is contained within a given volume of a substance. It is expressed in units of mass per unit volume, such as grams per cubic centimeter (g/cm³). In this example, we are given the mass of a material (48 grams) and its volume (6 cubic centimeters), and we can use the formula for density (density = mass / volume) to calculate its density. Plugging in the values, we get a density of 8 g/cm³, which means that 8 grams of the material occupy each cubic centimeter of space. The density of a material can provide important information about its properties, such as whether it will float or sink in a liquid, or how it will behave under different conditions.
Visit here to learn more about Density : https://brainly.com/question/29775886
#SPJ11
which list of particles is in order of increasing mass
Answers
The correct order of particles in increasing mass is option A) Electron, neutron, proton
The electron, being the lightest particle, has the smallest mass among the three. It weighs approximately 9.1 x 10^-31 kilograms. Neutrons, slightly heavier, have a mass of around 1.67 x 10^-27 kilograms. Protons, being the heaviest, have a mass of about 1.67 x 10^-27 kilograms.
In conclusion, the order of particles in increasing mass is electron, neutron, and proton. The electron, with the smallest mass, is followed by the neutron, and the proton is the heaviest among the three particles.
To know more about Electron, neutron, proton click here:
https://brainly.com/question/29248303
#SPJ11
The complete question is:
Which list of particles is in order of increasing mass:
A) Electron, neutron, proton
B) Neutron, electron, proton
C) Proton, electron, neutron
Brainpop bacteria true or false worksheet
Answers
Brainpop is an online educational resource that provides animated videos, interactive quizzes, and games on various subjects. One of the topics covered in Brainpop is bacteria, which are single-celled organisms that exist in different forms, some of which can cause disease.
To test the knowledge of students on bacteria, Brainpop offers a true or false worksheet that includes various statements related to bacteria. In this worksheet, students need to read each statement and determine whether it is true or false based on their understanding of the topic.
Some of the statements included in the Brainpop bacteria true or false worksheet are:
- Bacteria are always harmful to humans.
- Antibiotics can cure all types of bacterial infections.
- Bacteria can survive in extreme environments like the ocean floor and outer space.
- Bacteria can be helpful in some ways, like aiding in digestion and producing oxygen.
The correct answers to these statements are as follows:
- False. While some bacteria can cause disease, many are harmless or even beneficial to humans.
- False. Antibiotics are only effective against certain types of bacteria and are not effective against viruses.
- True. Bacteria are known to survive in extreme conditions, which makes them a fascinating subject of study for scientists.
- True. Bacteria play an essential role in various ecosystems and have many practical uses, such as in the production of food and medicine.
Overall, the Brainpop bacteria true or false worksheet is an excellent tool for students to test their knowledge and improve their understanding of bacteria. By answering these questions correctly, students can gain a better appreciation for the role that bacteria play in our lives and in the natural world.
For more such question organisms
https://brainly.com/question/30821424
#SPJ11
Note- The Question seems Incomplete, and complete question isn't available in the search engine.
what are the valency and valence electrons of
1) Calcium
2) Magnesium
3)Oxygen
4)Argon
Answers
Answer:
calcium - valency -: ( 2) valence electron -: 2magnesium - valency -:(2) valence electron -: 2 oxygen - valency. -: (2) valence electron -:. 8argon -. valency -:. (doesn't have because it is a noble gas so that it doesn't have valency). valence electron-:. 8.....Explanation:
hopes it help you a lot
pls. (mark me as brainlist ).......
the formula for caffeine is c8h10n4o2. how many total atoms are in 0.75 moles of caffeine
Answers
In 0.75 moles of caffeine, there are a total of 6 carbon atoms, 7.5 hydrogen atoms, 3 nitrogen atoms, and 1.5 oxygen atoms.
To determine the total number of atoms in 0.75 moles of caffeine, we need to consider the molecular formula of caffeine, which is C8H10N4O2. The molecular formula provides the ratios of each element present in the compound. By multiplying the number of atoms in each element by the corresponding coefficient in the molecular formula, we can calculate the total number of atoms. In this case, there are 8 carbon (C) atoms, 10 hydrogen (H) atoms, 4 nitrogen (N) atoms, and 2 oxygen (O) atoms in each molecule of caffeine. Multiplying these values by 0.75 moles will give us the total number of atoms in 0.75 moles of caffeine.
The molecular formula of caffeine, C8H10N4O2, provides the number of atoms for each element present in one molecule of caffeine. In this case, there are 8 carbon (C) atoms, 10 hydrogen (H) atoms, 4 nitrogen (N) atoms, and 2 oxygen (O) atoms.
To calculate the total number of atoms in 0.75 moles of caffeine, we need to multiply the number of atoms for each element by the coefficient in the molecular formula, and then multiply that by the number of moles (0.75 moles).
For carbon (C): 8 atoms x 0.75 moles = 6 atoms (since there are 8 carbon atoms in one molecule of caffeine).
For hydrogen (H): 10 atoms x 0.75 moles = 7.5 atoms (since there are 10 hydrogen atoms in one molecule of caffeine).
For nitrogen (N): 4 atoms x 0.75 moles = 3 atoms (since there are 4 nitrogen atoms in one molecule of caffeine).
For oxygen (O): 2 atoms x 0.75 moles = 1.5 atoms (since there are 2 oxygen atoms in one molecule of caffeine).
To learn more about molecular click here:
brainly.com/question/156574
#SPJ11
Draw atomic depictions similar to those in Problem 2.43 for:
(c) ⁷⁵₃₃As
Answers
The mass number of the element is 75, atomic number is 33 and number of neutron is 42.
What is Atomic Number ?The number of proton which is used to separate one element from the other element is called Atomic Number. It is denotes as symbol Z.
What is Atomic Notation ?
The atomic number is the number of proton which is present in the nucleus of an atom. It is represented as \(^{A}_{Z}X\).
where
X is the element
Z is the atomic number of that element.
A is the mass number of that isotope.
Now,
A = Z + N
75 = 33 + N
75 - 33 = N
42 = N
Thus from the above conclusion we can say The mass number of the element is 75, atomic number is 33 and number of neutron is 42.
Learn more about the Atomic Number here: https://brainly.com/question/487717
#SPJ4
The measure of the speed of the moving or
vibrating particles is called the
Answers
Answer:
Temperature
Explanation:
It's called temperature.
This is because, in any substance an increase in temperature means that the particles on the average will move with greater speeds, or that they have greater kinetic energy while a decrease in temperature means that the particles on the average will move with lesser speeds, or that they have lesser kinetic energy.
are you gay my fellow human being what ever your answer have a good day
Answers
Answer:
Yes, i'm gay.
Have a nice day!!
~Narancia
47.0 mL of an HBr solution were titrated with 37.5 mL of a 0.215 M LiOH solution to reach the equivalence point. What is the molarity of the HBr solution?
Answers
Answer:
0.172 M
Explanation:
M1V1=M2V2
(x)(47.0mL) = (37.5mL)(0.215M) solve for X
Answer: 0.172
Explanation:
How would I balance this?

Answers
The balanced chemical equation is as follows:
4KClO₃ ₍s₎ ⇒ 3KClO₄ ₍s₎ + 3KCl
What is balance chemical equation ?The term balanced chemical equation is defined an equation where the number of atoms of each type in the reaction is the equal on both reactants and product sides.
The chemical equation must be balanced in order to obey the law of conservation of mass. When the number of different atoms of elements in the reactants side equals the number of atoms in the products side, the chemical equation is balanced.
Thus, The balanced chemical equation is as follows:
4KClO₃ ₍s₎ ⇒ 3KClO₄ ₍s₎ + 3KCl
To learn more about the balanced chemical equation, follow the link;
https://brainly.com/question/28294176
#SPJ1
A donut has a density of 0.75 g/cm cubed and a mass of 100.0g. What is the volume of the donut?
Answers
Answer:
133.333333333 cm^3
Explanation:
Volume = Mass/Density
Name the elements that would have the following ground-state electron configurations: A. 1s^2 2s^2 2p^5 B. 1s^2 2s^2 2p^6 3s^2 C. 1s^2 2s^2 2p^6 3s^2 3p^3 D. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 E. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d
Answers
Fluorine (F) Neon (Ne) Phosphorus (P) Potassium (K) Calcium (Ca)
The electron configuration 1s^2 2s^2 2p^5 corresponds to the element fluorine (F) with atomic number 9.
The electron configuration 1s^2 2s^2 2p^6 3s^2 corresponds to the element neon (Ne) with atomic number 10.
The electron configuration 1s^2 2s^2 2p^6 3s^2 3p^3 corresponds to the element phosphorus (P) with atomic number 15.
The electron configuration 1s^2 2s^2 2p^6 3s^2 3p^6 4s^1 corresponds to the element potassium (K) with atomic number 19.
The electron configuration 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d corresponds to the element calcium (Ca) with atomic number 20.
The given electron configurations correspond to the following elements: A) Fluorine (F), B) Neon (Ne), C) Phosphorus (P), D) Potassium (K), and E) Calcium (Ca).
To learn more about atomic number, visit
brainly.com/question/11353462
#SPJ11
Determine the percent yield for the reaction between 46.5 g of ZnS and 13.3 g of oxygen if 18.4 g of ZnO is recovered along with an unknown quantity of sulfur dioxide (SO2).
Answers
If 18.4 g of ZnO and an unidentified amount of sulphur dioxide (SO2) are recovered from the reaction involving 46.5 g of ZnS and 13.3 g of oxygen, the percent yield is: 53.13%.
Explain about the Percent Yield?The majority of the time when conducting experiments, their results still wouldn't match the anticipated results. Errors or real-world issues that are not taken into consideration may result in losses. We could determine what percentage of the anticipated amounts were produced by the trials using the percent yield.First, the chemical reaction needs to be balanced.
ZnS + 1.5O₂ → ZnO + SO₂
Find limiting reactant.
= 46.5 grams of ZnS * (1 mol ZnS / 97.4 g of ZnS) * (32 g O₂ / 1 mol O₂)
= 22.9158 g of O₂
We required 22.9158 g of O₂ and 20.42 grams are given.
It makes O₂ limiting reactant.
Estimated mass of the ZnO recovered.
= 20.42 g of O₂ * (1 mol ZnO / 1.5 mol O₂) * (81.4 g of ZnO / 1 mol ZnO)
= 34.629 g ZnO
Theoretically, 34.629 grammes of ZnO were retrieved. The following equation can be used to get the percent yield:
Percentage yield = actual yield / theoretical yield * 100
Put the values;
Percentage yield = 18.4 / 34.629 *100
Percentage yield = 53.13%
Thus, if 18.4 g of ZnO and an unidentified amount of sulphur dioxide (SO2) are recovered from the reaction involving 46.5 g of ZnS and 13.3 g of oxygen, the percent yield is: 53.13%.
To know more about the Percent Yield, here
https://brainly.com/question/2451706
#SPJ1