calculate the stoichiometric ox-f mass ratio for the reaction between ch4 and o2. show the necessary step
Answers
The stoichiometric ox-f mass ratio for the reaction between CH4 and O2 is 1:2. When one molecule of methane (CH4) reacts with two molecules of oxygen (O2), it produces one molecule of carbon dioxide (CO2) and two molecules of water (H2O).
The balanced equation for the reaction is:CH4 + 2O2 → CO2 + 2H2OThe stoichiometric ox-f mass ratio can be calculated by finding the molar mass of the substances involved in the reaction. The molar mass of CH4 is 16.04 g/mol, and the molar mass of O2 is 32.00 g/mol.
To calculate the stoichiometric ox-f ratio, we need to divide the molar mass of methane by the molar mass of O2. This gives us : 16.04 g/mol ÷ 32.00 g/mol = 0.50125:1. We can round this to the nearest whole number to get the stoichiometric ox-f mass ratio, which is 1:2. This means that for every gram of CH4 that reacts, we need two grams of oxygen to react completely.
Know more about mass ratio here:
https://brainly.com/question/14577772
#SPJ11
Related Questions
SURELY SOMEONE HELP it’s urgent plllss I’ll brainlist u/5 star!!! answer the ones u know. :)
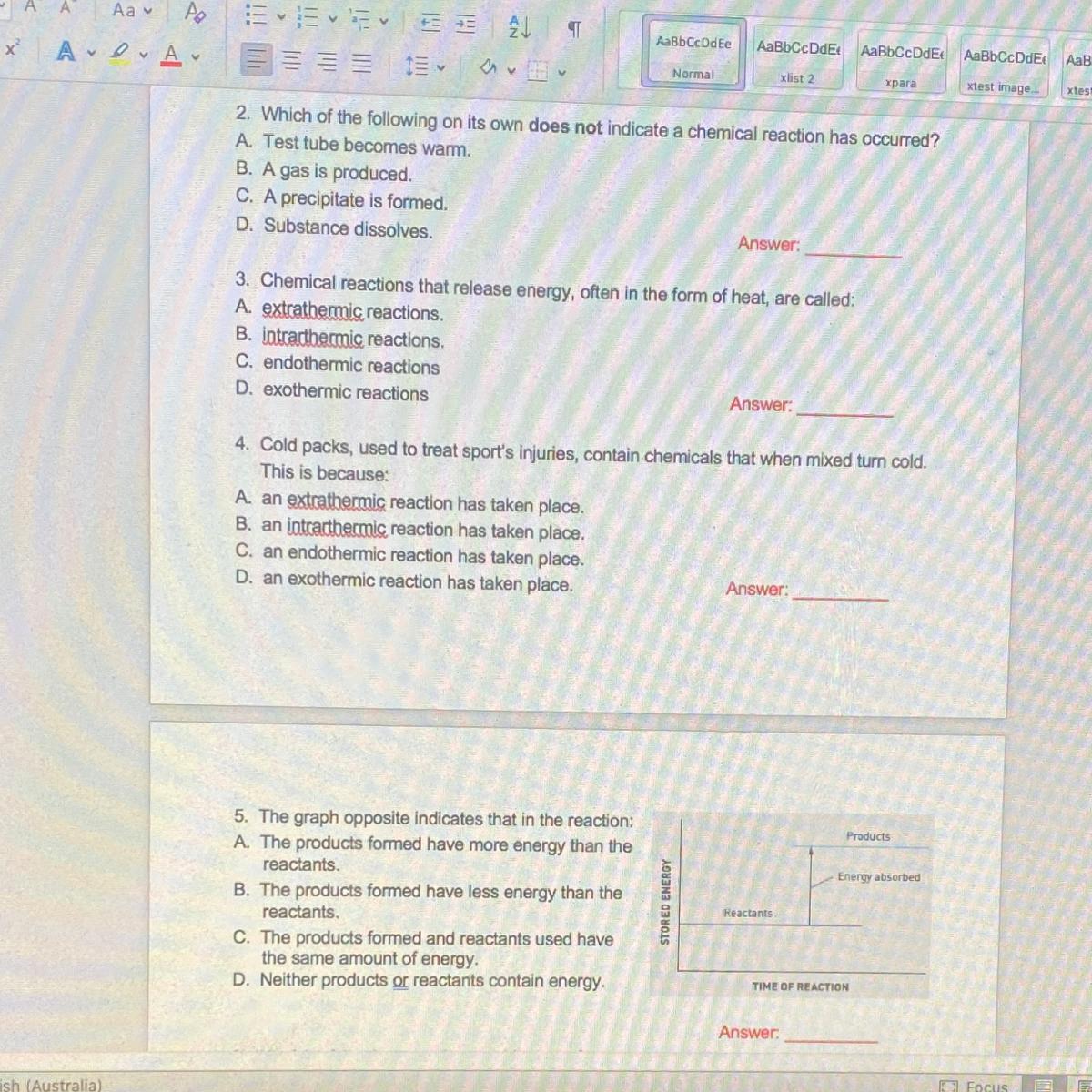
Answers
Answer:
3 exothermic reaction. only that much
Hi,
These are the answers.
• Question 2. C , Substance dissolves
• Question 3. D , Exothermic reaction
• Question 4. C , Endothermic reaction takes place
• Question 5. A , the products formed has more energy than reactants
Hope it helps you... pls mark brainliest if it helped you
Due Tomorrow!
What is [OH-] of a solution
with a pH of -1.0?
Answer in M
Answers
For chymotrypsin, which amino acid is involved in forming the oxyanion hole, but is not part of the catalytic triad?
Answers
The oxyanion hole is a structural feature found in many enzymes, including chymotrypsin, that stabilizes the transition state of the reaction and enhances catalytic efficiency.
The oxyanion hole is formed by a cluster of amino acid residues that interact with the carbonyl group of the substrate and facilitate its deprotonation during catalysis.
In chymotrypsin, the oxyanion hole is formed by the backbone amide groups of two amino acids: serine 195 and glycine 193. While serine 195 is part of the catalytic triad (together with histidine 57 and aspartic acid 102), glycine 193 is not. Instead, glycine 193 is located near the catalytic triad and helps to orient serine 195 in the correct position for substrate binding and catalysis. Together, the oxyanion hole and catalytic triad form a highly efficient system for cleaving peptide bonds in proteins.
To learn more about chymotrypsin refer to:
brainly.com/question/13638833
#SPJ4
If you have 60 moles of HCl, what should the volume of solution be to make a 10 M solution?
Answers
Answer:10
Explanation:
Multiply the volume by the density to get the mass.
Divide the mass by the molar mass to get the number of moles.
initial consentretion
Answers
What is initial concentration?
High initial concentration of spilled oil has a negative effect on the biotransformation process that causes a significant lag phase of about 2–4 weeks. Even after biostimulation, at least a week is needed for microorganisms to adapt and the entire bioremediation process may require months and even years to be completed (Atlas, 1995; Zahed et al., 2010a). The rates of uptake and mineralization of many organic compounds by a microbial population depend on the concentration of the compound (Olivera et al., 1997; Rahman et al., 2002).
Step by step explanation
For the reaction
if 5. 0 mol of CO2 are produced, how many moles of O2 were reacted?
a. None of these
b. 3. 3 mol
c. 12. 5 mol
d. 7. 5 mol
e. 6. 2 mol
Answers
If 5.0 mol of the CO₂ are produced, the number of the moles of the O₂ were reacted is 10 mol. The correct option is a. none of these.
The chemical equation is as :
CH₄ + 2O₂ → CO₂ + 2H₂O
The number of the moles of the CO₂ = 5 mol
The number of the moles of the CO₂ = mas / molar mass
The molar mass of the CO₂ = 44 g/mol
The 2 moles of the O₂ produced by the 1 mole of the CO₂
The number of the moles of the O₂ = 2 × 5 mol
The number of the moles of the O₂ = 10 mol.
The number of the moles of the O₂ required to produced 5 mol of the CO₂ is the 10 mol of the O₂. The correct option is a.
To learn more about moles here
https://brainly.com/question/19247935
#SPJ4
Which statement about light energy is incorrect?
A. Light energy travels as electromagnetic waves
B. We can only see light if the wavelengths are in a
certain part of the electromagnetic spectrum
C. Refraction is when light bends as it passes through
another medium
D. Light is absorbed at a higher rate if the color of the
object it hits is brighter
Answers
Answer:
The correct answer is C
Explanation:
light refracts whenever it travels at an angle into a substance with a different refractive index(optical density)
What happens to the carbonyl peak in a non-conjugated system relative to a conjugated one? Peak wavenumber shifts left by 60 Peak wavenumber shifts right by 30 Peak wavenumber shifts left by 30 Peak wavenumber shifts right by 60
Answers
The carbonyl peak wavenumber shifts to the left by 30 in a conjugated system compared to a non-conjugated one.
In an IR spectroscopy, the carbonyl peak is characterized by a strong absorption band between 1710-1730 cm-1. The carbonyl peak is due to the stretching of the C=O bond. In non-conjugated systems, the carbonyl peak has a wavenumber of 1710-1730 cm-1, but in conjugated systems, the peak shifts to the left by 30 cm-1 to a wavenumber of 1680-1700 cm-1. The reason for this shift is that in conjugated systems, the carbonyl group is delocalized, meaning that the electrons in the carbonyl double bond are spread out over a larger area. This delocalization reduces the strength of the C=O bond and causes the peak to shift to the left. This shift allows to distinguish between a non-conjugated and conjugated carbonyl compound.
To know more about carbonyl click below:
brainly.com/question/13278063#
#SPJ4
Restriction of which electrolytes is recommended in the management of high blood pressure?
Answers
Restriction of potassium electrolytes is recommended in the management of high blood pressure.
High blood pressure
a condition when the blood exerts too much pressure on the arterial walls.
Blood pressure beyond 140/90 is typically regarded as hypertension; blood pressure above 180/120 is regarded as severe hypertension.
Many times, high blood pressure goes unnoticed. If left untreated, it can eventually lead to illnesses like heart disease and stroke.
Blood pressure can be lowered by eating a less salty diet, moving frequently, and using drugs.
To learn more about the high blood pressure refer here:
https://brainly.com/question/1171823
#SPJ4
The Earth is in a protected area in the solar system where it does not get struck by asteroids.
True
False
Answers
Answer:
TRUEExplanation:
The ozone layer acts as a shield for life on Earth. Ozone is good at trapping a type of radiation called ultraviolet radiation, or UV light, which can penetrate organisms' protective layers, like skin, damaging DNA molecules in plants and animals.
In most mirrors, the virtual image appears to come from behind the mirror. True False
Answers
Answer
False
Explanation:
Which of the following instrument would you use to see a plant cell?
a. Kaleidoscope
C. Periscope
b. Microscope
D. Telescope

Answers
this is the answer .
Carbon dioxide molecules (select all that apply)
Group of answer choices
Protect the Earth from all of the harmful Ultraviolet (UV) radiation
Absorb most of the shortwave radiation emitted from the Sun
Are one of the most abundant constituents of Earth's atmosphere
Can move in many ways, thus absorbing and emitting infrared radiation
Answers
Carbon dioxide molecules can absorb and emit infrared radiation, and they are one of the most abundant constituents of Earth's atmosphere.
Thus, the correct options are:d) Are one of the most abundant constituents of Earth's atmospheree) Can move in many ways, thus absorbing and emitting infrared radiation
Carbon dioxide is a trace gas present in the Earth's atmosphere. It's a vital component of Earth's carbon cycle, which helps to regulate Earth's temperature and support life as we know it. Carbon dioxide molecules are one of the most common gases in the atmosphere, accounting for around 0.04% of the Earth's atmosphere.
The greenhouse effect is caused by carbon dioxide, methane, and other greenhouse gases. When the Sun's energy reaches the Earth's surface, it is absorbed and then radiated back into space as infrared radiation. Greenhouse gases absorb this radiation and trap it in the atmosphere, which causes the Earth's temperature to rise and the climate to change.
Carbon dioxide molecules are capable of absorbing and emitting infrared radiation due to their molecular structure, which consists of one carbon atom and two oxygen atoms. This property of carbon dioxide is the main reason it's classified as a greenhouse gas.
To know more about Carbon dioxide molecules visit:
https://brainly.com/question/12770212
#SPJ11
Consider the following list of salts (use information from your notes to help):
A) MgSO⁴
B) LiF
C) K²S
D) NH⁴CI
E) NaCH³COO
F) Csl
G) Cu(NO³)²
H) KBr
I) FeCl³
J) K²CO³
Which salts will generate an acidic solution?
Which salts will generate a basic solution?
Which salts will generate a neutral solution?
Answers
Salts that will generate an acidic solution: A) MgSO⁴, D) NH⁴CI, and G) Cu(NO³)².
Salts that will generate a basic solution: B) LiF, C) K²S, and J) K²CO³.
Salts that will generate a neutral solution: E) NaCH³COO, F) Csl, H) KBr, and I) FeCl³.
Which salts will result in different pH levels?Different salts can generate solutions with varying pH levels, indicating their acidic, basic, or neutral properties.
The salts MgSO⁴, NH⁴CI, and Cu(NO³)² will generate acidic solutions when dissolved in water. This is because they release hydrogen ions (H⁺) into the solution, increasing its acidity.
On the other hand, salts like LiF, K²S, and K²CO³ will produce basic solutions. They contain hydroxide ions (OH⁻) or produce hydroxide ions when dissolved, which increase the concentration of hydroxide ions and raise the pH, making the solution more alkaline.
Salts such as NaCH³COO, Csl, KBr, and FeCl³ will lead to neutral solutions as they do not significantly affect the concentration of H⁺ or OH⁻ ions when dissolved.
Learn more about salts
brainly.com/question/28102762
#SPJ11
To balance a chemical equation, it may be necessary to adjust the
A.coefficients.
B. subscripts.
c. formulas of the products.
D. number of products.
Answers
Answer:
A. Coefficients
Explanation:
that's the number in front of the molecules
When a rod of metal is heated intensely, its predominant color will
A) remain red as the intensity of light increases.
B) change from red through orange to white and then to blue.
C) change from blue through white, then orange, and finally red, when it becomes red-hot at its hottest.
D) be white, all colors mixed together, as the intensity of light increases.
Answers
When a rod of metal is heated intensely, its predominant color will change from red through orange to white and then to blue.
The process of heating changes the color of the rod of metal. In the beginning, it will appear red, followed by orange, white, and ultimately blue. A red-hot rod appears red when its temperature is relatively low. When the rod temperature increases, it will change colors, eventually becoming blue.
Change in the color of metal as the temperature increases:
Initially, when a rod of metal is heated, its temperature is relatively low. When a rod's temperature is about 500-800 degrees Celsius, it starts to glow red. When the temperature is increased, the metal becomes hotter, and the red color appears brighter. When the metal reaches 1000-1200 degrees Celsius, the red color changes to yellowish-orange. When the temperature is increased, the metal turns white hot, and its color changes to blue when the temperature reaches 1400 degrees Celsius or higher.
learn more about metal on
https://brainly.com/question/28650063
#SPJ11
A hot metal plate at 150°C has been placed in air at room temperature. Which event would most likely take place over the next few minutes?
A. Molecules in both the metal and the surrounding air will start moving at lower speeds.
b. Molecules in both the metal and the surrounding air will start moving at higher speeds.
c. The air molecules that are surrounding the metal will slow down, and the molecules in the metal will speed up.
d. The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.
Answers
Answer:
A
Explanation:
The molecules in both metal and surrounding air will start moving slower because of the sudden decrease in environment tmeperature. The thermal energy around the metal plate decreases, also decreasing the kinetic energy.
Which of the following molecules would have the highest boiling point?
a) hexane
b) octane
c) 2-propylpentane
d) 2-methylhexane
Answers
The molecule which would have the highest boiling point is 2-methylhexane. Thus, the correct option will be D.
What is boiling point?The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point of a liquid is a measure of its vapor pressure. The higher the boiling point, the higher the vapor pressure of the liquid, and the more heat is required to vaporize it.
The boiling point of a substance is affected by the strength and types of intermolecular forces. The stronger the intermolecular forces, the higher the boiling point. 2-methylhexane has highest boiling point because it has the highest number of carbons and branches, which contribute to its strong intermolecular forces that lead to a higher boiling point.
Therefore, the correct option is D.
Learn more about Boiling point here:
https://brainly.com/question/25777663
#SPJ11
a 250 gram sample of water at the boiling point had 35.0 kj of heat added. how many grams of water were vaporized? heat of vaporization for water is 40.6 kj/mole.
Answers
The required mass of water vaporized is 15.5 grams, from a 250 gram sample of water at the boiling point had 35.0 kj
Given: Mass of water (m) = 250 gHeat added (q) = 35.0 kJHeat of vaporization (ΔHvap) = 40.6 kJ/mole
To find:Mass of water vaporized (x) Formula:q = ΔHvap × nx = (q / ΔHvap) × nMass = moles × molar mass
We know that molar mass of water (H2O) = 18 g/molMoles of water vaporized (n) = (35.0 kJ / 40.6 kJ/mol) = 0.861 mol
Therefore,Mass of water vaporized (x) = 0.861 mol × 18 g/mol= 15.5
Detailed Solution: According to the given statement,250g of water was taken at its boiling point and 35.0 kJ of heat was added to it, we need to find how many grams of water were vaporized. To solve this question, first, we need to know the heat of vaporization for water, which is 40.6 kJ/mole. It means to vaporize 1 mole of water, 40.6 kJ of heat is required.
Mass of water (m) = 250 g Heat added (q) = 35.0 kJHeat of vaporization (ΔHvap) = 40.6 kJ/molen = q / ΔHvapn = (35.0 kJ / 40.6 kJ/mol) = 0.861 molMoles of water vaporized (n) = 0.861 mol
Therefore, Mass of water vaporized (x) = 0.861 mol × 18 g/mol= 15.5 g Hence, the required mass of water vaporized is 15.5 grams.
To learn more about mass visit;
https://brainly.com/question/11954533
#SPJ11
3. What does it look like when asteroids collide?
an explosión
a second moon
it is impossible to see
a comet
Answers
An explosión happens when asteroids collide.
When asteroids collide, little bits are expelled at orbital speeds that are slower than the original asteroids'.
What results from an asteroid collision?When asteroids collide, the ejected pieces have speeds that are low compared to the original asteroid's orbital speed. As a result, the pieces' orbits are near to one another, creating a "family" of smaller asteroids.
A massive impact between two asteroids may have caused the Earth to enter an ice age some 466 million years ago. The asteroid that was destroyed by the cosmic collision between Mars and Jupiter, which was around 93 miles (150 km) broad, was covered in a dense cloud of dust that travelled across the inner solar system.
The asteroids that might destroy the world if they struck the Earth are exceedingly uncommon. They would most likely need to be around a kilometre apart.
learn more about asteroids refer
https://brainly.com/question/11996385
#SPJ9
Which of the following pairs of solutions produces a precipitate when combined?
O NH4Cl and AgNO3
O Na₂SO4 and KCI
O Fe(NO)3 and KCI
O KOH and NH4CI
Answers
Answer:
Answer is NH4Cl and AgNO3
Explanation:
:)
The investigation that makes it hard to control variables is
.
Answers
The investigation that makes it hard to control variables is an observational study.
What is an observational study?
When the independent variable is not under the researcher's control due to ethical considerations or logistical limitations, an observational study infers information from a sample to the community.
The main distinction between observational studies and experimental designs is that the answers of participants are unaffected, whereas in experiments at least some participants are randomly assigned to receive a treatment condition.
Learn more about an observational study at: https://brainly.com/question/14393640
#SPJ1
ASAP HELP WILL MARK BRAINLIST 7,8,9, and 10

Answers
8. c. 75 g/ml
9. b. .25g/cm3
10. b. the density decreases
How many grams of H2 can be produced from the reaction of 11.50 g of sodium with an excess of water? The equation for the reaction is 2 Na + 2 H2O -> 2 NaOH + H2 Ans: 0.504 2 g H2 I would like to know how to solve this problem, the teacher gave me the answer but I am unsure how to solve it thanks!
Answers
Answer:
\(m_{H_2}=0.504gH_2\)
Explanation:
Hello,
In this case, since the reaction is:
\(2 Na + 2 H_2O \rightarrow 2 NaOH + H_2\)
We notice that since there is an excess of water, we can directly compute the yielded grams of hydrogen by using the following stoichiometric procedure, considering the 2:1 molar ratio between sodium and hydrogen (notice the 2 before the sodium and the 1 before the hydrogen at the chemical reaction) and that gaseous hydrogen has a molar mass of 2 g/mol:
\(m_{H_2}=11.50gNa*\frac{1molNa}{22.98gNa} *\frac{1molH_2}{2.02molNa} *\frac{2gH_2}{1molH_2} \\\\m_{H_2}=0.504gH_2\)
Best regards.
Answer:
0.503g of H₂ are produced
Explanation:
Based on the chemical reaction:
2 Na + 2 H₂O → 2 NaOH + H₂
2 moles of Na react with 2 moles of water to produce 2 moles of NaOH and 1 mole of H₂
11.50g of Na -limiting reactant, molar mass 22.99g/mol- are:
11.50g× (1mol / 22.99g) = 0.500 moles of Na.
As 2 moles of Na produce 1 mole of H₂:
0.500 moles of Na × (1 mole H₂ / 2 moles Na) = 0.250 moles of H₂
As molar mass of H₂ is 2.01g/mol:
0.250 moles of H₂ × (2.01g / 1mol) = 0.503g of H₂ are produced
When water is hydrolyzed in the presence of NaCl, the possible half-cell equations are given in the accompanying table. Experimentally, it is found that H2 (g) and Cl2 (g) are formed, while O2 (g) and Na (s) are not. What is the best explanation for this?
Answers
The formation of H2 (g) and Cl2 (g) during hydrolysis in the presence of NaCl is due to the difference in reduction potentials of the half-cell equations and the selective reduction and oxidation of the ions present in the solution.
The best explanation for the formation of H2 (g) and Cl2 (g) during the hydrolysis of water in the presence of NaCl lies in the standard reduction potentials of the half-cell equations given in the accompanying table.
It can be observed that the reduction potential of Cl2 is much higher than that of O2, and the reduction potential of H2 is much lower than that of Na. This means that Cl2 has a stronger tendency to be reduced than O2, and H2 has a weaker tendency to be oxidized than Na.
During hydrolysis, the water molecule is split into H+ and OH- ions. The H+ ions are reduced by the Cl- ions to form HCl, which then reacts with more H+ ions to form H2 gas.
The OH- ions are oxidized by water molecules to form O2 gas and more OH- ions. The Na+ ions, however, have a higher tendency to be oxidized than the water molecule, so they do not participate in the reaction.
To know more about hydrolysis refer here:
https://brainly.com/question/29439050#
#SPJ11
A bottles contains 3.100 mL of a liquid. The mass of the liquid is 2.060 g. What is the density of the liquid? (BE SURE TO
SHOW ALL THREE PARTS: EQUATION, WORK, AND PROPER FULL ANSWER.
Answers
Answer:
0.66 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question we have
\(density = \frac{2.06}{3.10} \\ = 0.664516..\)
We have the final answer as
0.66 g/mLHope this helps you
The fictional element, marvellium has 4 isotopes with mass numbers of 21, 22,24, and 28 amu. Each isotope is found in equal amounts in nature. What number is closest to the average atomic mass of marvellium
Answers
Answer:
The answer to that question is 24 AMU.
Explanation:
I know this because i got the answer correct on the quiz. To get the average, you just add the 4 atomic masses given to you together then divide by 4.
Which type of bond exists in each compound?
a) kclkcl ionic bonds
b) nonpolar covalent bonds
c) polar covalent bonds
d) bcl3bcl3 nonpolar covalent bonds
e) polar covalent bonds ionic bonds
Answers
a) KCl: Ionic bond - KCl exhibits ionic bonding due to the transfer of electrons from potassium to chlorine, resulting in the formation of K+ and Cl- ions.
b) Nonpolar covalent bonds (specific compound not mentioned) - The bond type cannot be determined without specifying the compound, as nonpolar covalent bonds occur when electrons are shared equally between atoms.
c) Polar covalent bonds (specific compound not mentioned) - The bond type cannot be determined without specifying the compound, as polar covalent bonds arise when there is an unequal sharing of electrons, resulting in partial charges.
d) BCl3: Nonpolar covalent bonds - BCl3 exhibits nonpolar covalent bonds because boron and chlorine have similar electronegativities, resulting in equal electron sharing.
e) Polar covalent bonds The bond type cannot be determined without specifying the compound, as polar covalent bonds occur when there is an unequal sharing of electrons, resulting in partial charges
a) KCl: Ionic bond
Ionic bonds exist between K+ and Cl- ions in KCl. Ionic bonds are formed between a metal cation (K+) and a nonmetal anion (Cl-) through the transfer of electrons.
b) Nonpolar covalent bonds
Nonpolar covalent bonds are characterized by equal sharing of electrons between atoms. The compound mentioned is not specified, so we cannot determine the exact compound that exhibits nonpolar covalent bonds.
c) Polar covalent bonds
Polar covalent bonds occur when there is an unequal sharing of electrons between atoms, resulting in partial charges. The compound mentioned is not specified, so we cannot determine the exact compound that exhibits polar covalent bonds.
d) BCl3: Nonpolar covalent bonds
BCl3 (boron trichloride) exhibits nonpolar covalent bonds. In BCl3, boron (B) forms three single covalent bonds with chlorine (Cl) atoms. The bonds are nonpolar since boron and chlorine have similar electronegativities, resulting in equal sharing of electrons.
e) Ionic bonds
Ionic bonds exist between oppositely charged ions. The compound mentioned is not specified, so we cannot determine the exact compound that exhibits ionic bonds.
For more such question on Ionic bond visit:
https://brainly.com/question/977324
#SPJ8
consider the equilibrium of each of the carbonyl compounds with hcn to produce cyanohydrins. which is the correct ranking of compounds in order of increasing keq for this equilibrium
Answers
The correct ranking of compounds in order of increasing keq for this equilibrium is Acetone < Propanone < Benzaldehyde.
The correct ranking of compounds in order of increasing keq for the equilibrium of each of the carbonyl compounds with HCN to produce cyanohydrins is
Acetone < Propanone < Benzaldehyde.
In the above reaction, the cyanide ion acts as a nucleophile and attacks the carbonyl carbon atom in the carbonyl compound to form an intermediate compound. Then the intermediate compound formed by the reaction between HCN and the carbonyl compound undergoes an intramolecular rearrangement to form cyanohydrin.Based on the stability of intermediate compound formed, the order of increasing stability is as follows:
Acetone < Propanone < Benzaldehyde.
Since the keq is directly proportional to the stability of the intermediate, the order of increasing keq for the equilibrium of each of the carbonyl compounds with HCN to produce cyanohydrins is
Acetone < Propanone < Benzaldehyde.
The correct ranking of compounds in order of increasing keq for this equilibrium is
Acetone < Propanone < Benzaldehyde.
To know more about Benzaldehyde visit:
https://brainly.com/question/29754365
#SPJ11
HELPPPP IM GIVING 95 POINTS FRICKING HURRY

Answers
Answer:
C
Explanation:
As we can see here the precipitation spikes in June and the evaporation amount starts to increase. Let's test these 4:
A:
'The lake level will drop because there is more evaporation than precipitation'
As we can see, there is more precipitation than evaporation, so A is not correct.
B:
The lake level will drop because there is more precipitation than evaporation
Evaporation means that water (literally) disappears into thin air, so B would not be correct, as precipitation raises the water level
C:
The lake level will rise because there is more precipitation than evaporation
This is the correct answer, since 1) there is more precipitation than evaporation in June and 2) it makes sense that the lake level will rise with more precipitation.
There we have it! The answer is: C