Calculate the volume occupied by 25 g of co 2 at 0.84 atm and 25°c.
Answers
Answer:
12.4 L.
Explanation:
To calculate the volume occupied by 25 g of CO2 at 0.84 atm and 25°C, we can use the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
First, we need to find the number of moles of CO2 present:
n = m / M
where m is the mass of CO2 and M is the molar mass of CO2. The molar mass of CO2 is 44.01 g/mol.
n = 25 g / 44.01 g/mol
n ≈ 0.568 mol
Next, we can plug in the values for P, n, R, and T to find the volume:
V = nRT / P
V = (0.568 mol) (0.08206 L·atm/mol·K) (298 K) / (0.84 atm)
V ≈ 12.4 L
Therefore, the volume occupied by 25 g of CO2 at 0.84 atm and 25°C is approximately 12.4 L.
Mass of 25 g of CO₂ occupies a volume of 12.9 L at 0.84 atm and 25°C.
The volume occupied by 25 g of CO₂ at 0.84 atm and 25°C can be calculated using the ideal gas law:
PV = nRT
where P is the pressure in atmospheres, V is the volume in liters, n is the number of moles, R is the gas constant (0.0821 L·atm/K·mol), and T is the temperature in Kelvin.
First, we need to convert the mass of CO₂ to the number of moles. The molar mass of CO₂ is 44.01 g/mol, so:
n = m/M = 25 g / 44.01 g/mol = 0.567 mol
Next, we can plug in the values into the ideal gas law equation:
V = nRT/P = (0.567 mol)(0.0821 L·atm/K·mol)(298 K) / 0.84 atm
V = 12.9 L
It's important to note that the temperature must be converted to Kelvin (25°C + 273 = 298 K) for the equation to work, and the pressure must be in atmospheres (0.84 atm). Also, we assume that CO₂ behaves as an ideal gas under these conditions.
To know more about ideal gas law click on below link:
https://brainly.com/question/28257995#
#SPJ11
Related Questions
Demonstrate that kgL^1 and gcm^3 are
equivalent units of density
Answers
1 kg = 1000g
1l = 1000cm3
therefor
kg/l = 1000g/1000cm3
cancel the zeros
kg/l = g/cm3 demonstrated
A student proposed the Bohr model below for sodium (Na). Is this student’s model correct? Justify your answer

Answers
The Bohr model is a representation of the electronic configuration of the atom. According to this model, each energy level can hold a certain number of electrons. In the first energy there can only be 2 electrons, in the second and the following energy levels there can be a maximum of 8 electrons.
Sodium, Na which has 11 electrons in total so in the first level it will have two electrons, in the second level it will have 8 electrons and in the third level it will have the missing electron.
In the model proposed by the student, the electrons are represented in blue. The model proposed by the student is incorrect.
We see that in the second energy level he drew 9 electrons, this is incorrect since from the second energy level there can only be 8 electrons, the remaining electron must be located in the third energy level.
cd ni2 →cd2 ni what is the standard cell potential of a nickel-cadmium electrochemical cell?
Answers
The reaction you provided shows the oxidation of cadmium and the reduction of nickel.
To calculate the standard cell potential of a nickel-cadmium electrochemical cell, we need to use the standard reduction potentials of both elements. The standard reduction potential of nickel is -0.25 V, and the standard reduction potential of cadmium is -0.40 V.
Using the equation:
E°cell = E°reduction (cathode) - E°reduction (anode)
We can calculate the standard cell potential of the nickel-cadmium electrochemical cell as:
E°cell = -0.25 V - (-0.40 V) = 0.15 V
Therefore, the standard cell potential of a nickel-cadmium electrochemical cell is 0.15 V.
To know about reaction:
https://brainly.com/question/30464598
#SPJ11
Question 5 (Mandatory) (1 point) For the reaction shown, how many grams of oxygen will be required to react with 15.5 grams of chromium?4 Cr + 3 O2 --------> 2 Cr2O3Question 5 options:A) 12.7 g O2B) 21.5 g O2C) 14.3 g O2D) 7.15 g O2Question 6 (Mandatory) (1 point) Consider the following combustion reaction: 2 C4H10 + 13 O2 ------------> 8 CO2 + 10 H2OIf 5.05 grams of C4H10 reacts with more than enough of O2, what is the maximum amount of CO2 that can be formed?Question 6 options:A) 122 g CO2B) 15.3 g CO2C) 0.956 g CO2D) 61.2 g CO2Question 7 (Mandatory) (1 point) Consider the reaction between HCl and O2: 4 HCl + O2 ---------------> 2 H2O + 2 Cl2Calculate the theoretical yield of H2O when 64 grams of HCl react with 19 grams of O2.Question 7 options:A) 16 g H2OB) 21 g H2OC) 32 g H2OD) 43 g H2OQuestion 8 (Mandatory) (1 point) Consider the following chemical reaction: 4 Al + 3 O2 ---------------> 2 Al2O3If 12.85 g of Al reacts completely with oxygen and 18.42 g of aluminum oxide is obtained, what is the percent yield of the reaction?Question 8 options:A) 24.28%B) 75.86%C) 65.91%D) 69.76%Question 9 (Mandatory) (1 point) When a mole of gaseous propane (C3H8) reacts completely with oxygen gas, 2044 kJ of energy is formed, along with gaseous carbon dioxide and water vapor. Which of the following equations is a correct representation of this thermochemical reaction?Question 9 options:A) C3H8(g) + O2(g) -----------> CO2(g) + H2O(g) ΔH = -2044 kJB) C3H8(g) + O2(g) -----------> CO2(g) + H2O(g) + 2044 kJC) C3H8(g) + 5 O2(g) + 2044 kJ -----------> 3 CO2(g) + 4 H2O(g) D) C3H8(g) + 5 O2(g) -----------> 3 CO2(g) + 4 H2O(g) + 2044 kJQuestion 10 (Mandatory) (1 point) The evaporation of water is endothermic: H2O(l) + 44.01 kJ --------> H2O(g) If 275 kJ of heat is absorbed, what mass of water will evaporate?Question 10 options:A) 0.347 gB) 2.18 x 105 gC) 113 gD) 6.25 gQuestion 11 (Mandatory) (1 point) Based on the following reaction: BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq) If a reaction mixture contains 4.16 g of BaCl2 and 3.30 g of Na2SO4 how many moles of the precipitate will be formed?Question 11 options:A) 0.178 molesB) 0.0200 molesC) 0.0241 molesD) 0.0278 molesQuestion 12 (Mandatory) (1 point) The following chemical equation, which is unbalanced, shows the reaction for the rusting of iron: Fe(s) + O2(g) → Fe2O3(s)How many moles of O2 are required to react completely with 1.396 g of Fe?Question 12 options:A) 0.00937 moles of O2.B) 0.0187 moles of O2.C) 0.00469 moles of O2.D) 0.0375 moles of O2.Question 13 (Mandatory) (1 point) A reaction mixture of 18.215 g Na2CO3 and 10.938 g HCl react according to the following balanced equation: Na2CO3 + 2HCl → 2NaCl + H2O + CO2What is the theoretical yield for NaCl, in moles, from this reaction mixture?Question 13 options:A) 0.300 moles of NaClB) 0.172 moles of NaClC) 0.150 moles of NaClD) 0.344 moles of NaClQuestion 14 (Mandatory) (1 point) A reaction mixture of 0.1400 g of NaOH and 0.1400 g HCl reacts according to the following equation: NaOH + HCl → NaCl + H2O ∆H = -31.0 kJWhat is the heat change, q, corresponding to this reaction mixture?Question 14 options:A) 0.109 JB) -0.119 JC) -119 JD) -109 JQuestion 15 (Mandatory) (1 point) A mass of 0.6539 g of zinc is dissolved in excess hydrochloric acid, according to the following equation: Zn(aq) + 2HCl(aq) → ZnCl2(aq) + H2(g) How many moles of Cl- ions are formed in the reaction?Question 15 options:A) 0.002 molesB) 0.02 molesC) 0.01 molesD) 0.1 moles
Answers
Question 5
Answer
D - 7.15 g O2
Explanation
Given:
Balanced chemical equation:
\(4Cr\text{ + 3O}_2\rightarrow2Cr_2O_3\)mass of Cr = 15.5 g
Required: mass of Oxygen
Solution:
Step 1: Find the moles of Cr
n = m/M where n is the number of moles, m is the mass and M is the molar mass
n = 15.5 g/51,9961g/mol
n = 0.298 mol
Step 2: Use stoichiometry to find the moles of O
The molar ratio between Cr and O is 4:3
Therefore the number of moles of O = 0.298 x (3/4) = 0.224 mol
Step 3: From the moles, we can find the mass of O
m = n x M
m = 0.224 x 31.998
m = 7.168 g which is closer to D = 7.15 g
A sample of certain gas have Volume of 1.25 L ATM _125 degree Celsius and5.0 ATM the gas is compressed 50.0 ATM a volume of 325 mL. what is final temperature?
Answers
The final temperature of the gas is approximately 40.96 Kelvin.
To determine the final temperature of the gas, we can use the ideal gas law, which states:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature in Kelvin.
First, let's convert the given temperatures to Kelvin. We have:
Initial temperature: -125 degrees Celsius = 148 K (approximate)
Final temperature: Unknown
The initial conditions of the gas are as follows:
Initial pressure (P1) = 1.25 atm
Initial volume (V1) = 1250 mL = 1.25 L (since 1 L = 1000 mL)
Initial temperature (T1) = 148 K
The final conditions of the gas are as follows:
Final pressure (P2) = 50.0 atm
Final volume (V2) = 325 mL = 0.325 L
Final temperature (T2) = Unknown
Using the ideal gas law, we can set up the following equation:
(P1 * V1) / T1 = (P2 * V2) / T2
Substituting the known values:
(1.25 atm * 1.25 L) / 148 K = (50.0 atm * 0.325 L) / T2
Simplifying the equation:
T2 = (50.0 atm * 0.325 L * 148 K) / (1.25 atm * 1.25 L)
T2 = 40.96 K
For more such questions on temperature visit;'
https://brainly.com/question/4735135
#SPJ8
product of molar concentration of ion raised to the power of number of ions produced per compound in saturated solution is called as
Answers
The product of molar concentration of ion present in chemical reactants raised to the power of number of ions produced per chemical compound in a saturated solution is called: solubility product.
What is solubility product?Solubility product can be defined as a product of the molar concentration of ion present in two or more chemical reactants raised to the power of number of ions produced per chemical compound in a saturated solution.
The formula for solubility product.Mathematically, the solubility product is given by this formula:
\(K_{sp} = [A^+]^x[B^{-}]^y\)
Where:
k is the rate constant.A is the molar concentration of ion.B is the molar concentration of ion.x and y are the number of ions.In Chemistry, the solubility product is typically used as an equilibrium constant for the dissolution of a chemical compound in a saturated solution.
Read more on solubility product here: https://brainly.com/question/9905859
What term describes the smallest unit that can perform all of the functions necessary for life
Answers
Answer:
Cell
A cell is the smallest unit of a living thing. Cells are the basic building blocks of all organisms
which of the following describes a liquid

Answers
Answer:
b I beleive
Explanation:
the shape of a liquid changes based on the container it is in, but the volume stays the same
Answer:
Option B
Explanation:
The valence electrons found in metallic bonds are different from other bonds
because
A. their valance electrons are free-roaming
B.they allow conductivity of electricity
C.their valence electrons are immobile
D. They’re able to share electrons with other atoms
Answers
Answer:
their valence electrons are free roaming
2+2+2 plssssssssssssssssssssssssss
Answers
Answer:
6
Explanation:
2+2+2=6 :)
Answer:
6
Explanation:
vvvffhjh DC hcf vhcnbfhbc
Solve for the Gradient AB. The units will be in m/km but you should not
leave units in the answer because the answer key will not take them. Write
the answer to the tenths place and no units.
.X
B
Lake
-100
Blue River
50
А
D
5 kilometers
Contour interval 10 meters
N
0
2
3
5 kilometers
Answers
Answer:
help me mark me as a brinliest
Which of the following could form an ionic bond with an anion
Answers
Answer:
A. (Hg2^2+)
Explanation:
Just took the review on Edge! :D
er or cooler?
Is energy created during an exothermic
reaction? Explain.
HELPP ASAP
Answers
for the measurements 0.123 m, which digits is the estimated digit?
Answers
Answer:
aaaaaaaaaaaaa
Explanation:
aaaaaaaaaaaaaaaa
How many grams of sodium chloride could be produced from 94.3 g of sodium
Answers
239.8 g so 240g
Explanation:
plz let me know if this is right than I can tell u how I did it :)
Each night you measure your height just before going to bed. When you arise each morning, you measure your height again and consistently find that you are 1 inch taller than you were the night before but only as tall as you were 24 hours ago. Is what happens to your body in this instance best described as a physical change or a chemical change?
a. chemical change because it involves changes in your bone structure
b. chemical change because it involves your body
c. physical change because water expands as it freezes
d. physical change because it readily reverses
Answers
The best answer to this question is
d. physical change because it readily reverses
The observed phenomenon of being 1 inch taller in the morning and returning to the previous height by the end of the day is primarily due to the compression and decompression of the spinal discs in the human body. Throughout the day, as you go about your activities and bear weight on your spine, the discs between the vertebrae compress. This compression leads to a slight decrease in height. When you lie down and sleep at night, the spinal discs have a chance to decompress, and as a result, you regain the height lost during the day.
This change is classified as a physical change rather than a chemical change because it does not involve any alterations in the chemical composition or structure of the substances involved. The change in height is purely a result of the physical properties and behavior of the spinal discs. It is a reversible process because the compression and decompression of the discs can occur repeatedly, leading to a temporary change in height on a daily basis.
Therefore, option d is the most appropriate choice because it correctly describes the nature of the observed change and its reversibility.
Learn more about readily ,visit;
https://brainly.com/question/31690056
#SPJ11
Is this equation equal? Why or Why not?
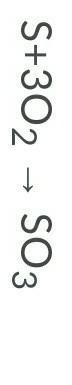
Answers
The next level of organization is the _____.
Answers
Answer:
The highest level of organization for living things is the biosphere; it encompasses all other levels. The biological levels of organization of living things arranged from the simplest to most complex are: organelle, cells, tissues, organs, organ systems, organisms, populations, communities, ecosystem, and biosphere.
Hopefully this helps feel free to mark brainliest :)
_____ A molecule is the smallest part of
A an element
B a compound
C a substance
D an atom
Answers
Answer:
B.a molecule is the smallest part of a compound
Answer:
substance
Explanation:
when carbon is heated in a limited supply of oxygen, a gas is obtained.
1 .what is the name of this gas
Answers
Answer:
carbon monoxide
I think its correct but I am not sure
Answer: when carbon is heated in air carbon dioxide is formed, so is incomplete combustion which results in carbon monoxide.
Explanation: but when carbon dioxide reacts with more oxygen carbon monoxide is formed i guess.
I AM A BIT SURE BUT HOPE THIS HELPSSSS!!!!
which chemical reaction absorbs energy?
A. photosynthesis
B. explosion
C. current produced by a battery
D. combustion of fuels
E. activated hand warmers
Answers
Answer:
A is your answer
which of the following is an accurate description of a coenzyme? multiple choice question. small inorganic ions that temporarily attach to the outside of an enzyme and promote a chemical reaction small molecules permanently attached to the outside of an enzyme that aid in catalysis organic molecules that temporarily attach to an enzyme and promote a chemical reaction without being changed during the reaction
Answers
Answer:
None of the options in the multiple choice question accurately describes a coenzyme.
A coenzyme is an organic molecule that works together with an enzyme to facilitate a chemical reaction. It is not permanently attached to the enzyme, but instead binds temporarily to the enzyme's active site, often through non-covalent interactions.
Coenzymes are important in many biological processes, including metabolism and energy production, and they often act as carriers of electrons or functional groups between different enzymes or biochemical pathways.
Examples of coenzymes include vitamins such as niacin (which is part of the coenzyme NAD+) and riboflavin (which is part of the coenzyme FAD). Coenzymes can also be derived from other molecules, such as coenzyme A (derived from the vitamin pantothenic acid), which plays a key role in many metabolic reactions.
4. A sample of oxygen gas has a volume of 150 mL when its pressure is 114 kPa. If the pressure is
standard pressure and the temperature remains constant, what will the new gas volume be?
Answers
Answer: 169ml
Explanation
At constant temperature, PV is constant
Standard pressure is 101 kpa
150ml X 114kpa=?mlX101 kpa
?=114/101 X150 = 169 ml
The calculation of new volume using Boyle's law. P₁V₁= P₂V₂, the new volume is 169mL.
What is Boyle's law?The pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; i.e., in equation form, pv = k, a constant.
From given data.
oxygen gas has a volume of 150 mL
Standard pressure is 101 kpa
At 114kPa the volume is = x
P₁V₁= P₂V₂
150ml X 114kpa=xml X101 kpa
x=114/101 X150
= 169mL
To find more about STP, refer the link below:
brainly.com/question/14004233
#SPJ2
1. Even though Ecuador and Norway are located in the northern hemisphere, Norway is colder compared to Ecuador. Explain.
Answers
Norway is a Nordic nation in North-Western Europe that borders Sweden, Finland, and Russia. Due to its reputation for having an exceptionally cold winter season, it has grown to be a very famous tourist destination in Europe, yet more than half of its yearly tourists arrive during the milder months of May to August.
The lowest temperature ever recorded in Norway was -51.4°c on New Year's Day in 1886. Although temperatures and meteorological conditions around the world vary, Norway may experience some rather extreme lows. Norway has a variety of climatic characteristics that explain why it has the coldest climate in all of Europe. Here are a couple of them justifications.
Learn more about Norway here-
https://brainly.com/question/28480874
#SPJ9
Plss answer and I will give the brainliest.
If a typical aluminum beverage can weighs 13.5 grams, how much alum could you produce for every 210 cans collected. Show your work, based on your own experiment.
The following is what I got from my experiment.
- mass of aluminum can: 0.4996 grams
- mass of alum produced:- 5.183 grams
Molar mass of aluminum: 26.98 grams
Answers
The alum produced during experiment for every 210 can is 38.21 kg
What is experiment?Experiment can be defined as a technique used to establish or refute a hypothesis, or to determine the efficacy or likelihood of anything new.
It can also be defined as a series of acts and observations carried out in the context of attempting to solve a specific problem or question.
According to your experiment 0.4996 gram of aluminum will produce 5.183 grams of alum.
Mass of 1 aluminum can = 13.5 g
Mass of 210 can = 13.5 x 210 = 2835g
Mass of alum produced by one gram of aluminum = 13.48 g
Mass of alum produced by 2835 g = 13.48 x 2835
= 38.21 kg
The amount of alum produced by 210 can = 38.21 kg
Thus, the alum produced during experiment for every 210 can is 38.21 kg
To learn more about experiment, refer to the link below:
https://brainly.com/question/11256472
#SPJ1
The VSEPR theory allows us to determine the Select one: A. charge on an ion B. color of a compound C. bond type for a molecule D. shape of a molecule E. formula for a compound
Answers
We are able to determine a molecule's shape using the VSEPR theory.
What is the VSEPR chemical bonding theory?The VSEPR theory assumes that the molecular shape minimizes these repulsions and that all electron pairs, including bonding pairs and lone pairs, repel one another, particularly when they are close to one another.
How does VSEPR theory work?The molecule's geometry can be determined with the help of VSEPR theory. As indicated by the VSEPR hypothesis, the electrons repulse each other and will, consequently, take on a game plan that limits this repugnance. When the electron pairs or groups of electron pairs are as far apart as possible, repulsions are at their lowest.
To know more about molecule's visit :-
https://brainly.com/question/30465503
#SPJ1
Does a hypothesis explains what the scientist thinks will happen during the experiment.
Answers
Hope this helps!
The molecular mass of a compound is 30 u. The percent composition of carbon and hydrogen in compound is 80% and 20% respectively, then the
ratio of molecular mass to empirical formula mass of the compound is
01:1
021
031
04-1
Answers
The ratio of molecular mass to empirical formula mass of the compound is 2:1.
The correct option is B.
What does molecular mass mean?The mass of a certain molecule is its molecular mass, which is measured in daltons. Because they contain various isotope of an element, multiple molecules of the same compound can have distinct molecular weights.
Briefing:Mass of C = 80g
Mass of H = 20 g
no. of moles of C
= 80/12
= 6.66
No. of moles of H
= 20/1
= 20
Ratio Carbon = 6.66/6.66 =1
Hydrogen = 20/6.66 = 3
Empirical formula = CH₃
=12 + 3 * 1
= 12+3
= 15
Molecular mass to empirical formula ratio =
30/15 = 2
To know more about Molecular mass visit:
https://brainly.com/question/15880821
#SPJ9
Find the half life of hydrogen-3 if 240,000 H-3 nuclei decay to 15,000 nuclei in 48 days.
Answers
Half-life of hydrogen-3 if 240,000 H-3 nuclei decay to 15,000 nuclei in 48 days is calculated as to be 12 days.
What is meant by half life?The amount of time it takes for half of the initial number of nuclei to decay is called as half life.
N = N₀ (1/2)^(t/T)
N₀ is initial number of nuclei
N --> number of nuclei left after time t
T is half-life of the substance
N₀ = 240,000; N = 15,000 ; t = 48 days
15,000 = 240,000 (1/2)^(48/T)
1/16 = (1/2)^(48/T)
(1/16) = ㏒[(1/2)^(48/T)]
So ㏒(1/16) = (48/T) ㏒g(1/2)
T = -48 / ㏒(1/2) * ㏒(1/16)
T = 12 days
Therefore, half-life of hydrogen-3 is 12 days.
To know more about half life, refer
https://brainly.com/question/25750315
#SPJ1
The distance to the moon is 238,855 miles. How many meters is this? Record your answer in scientific notation.
Answers
Answer:
384399861 meters
Explanation: