Chemicals in a lab kit may potentially cause _____ if mishandled or consumed. burns serious illness death All of the above
Answers
Chemicals in a lab kit may potentially cause all of the following if mishandled or consumed: burns, serious illness, and death.
Here is a step-by-step breakdown:
Burns:
Many chemicals used in laboratories are corrosive or can react violently with certain substances. If mishandled, they can cause burns to the skin or eyes. This can occur through direct contact or exposure to fumes or splashes.
Serious Illness:
Some chemicals are toxic and can lead to serious illness if inhaled, ingested, or absorbed through the skin. Toxic chemicals can cause damage to organs, disrupt bodily functions, or have long-term health effects. Symptoms of chemical exposure can vary depending on the specific substance involved.
Death:
In extreme cases, mishandling or consuming certain chemicals can be fatal. Highly toxic or reactive substances, if not handled properly, can lead to severe injury or even death. This emphasizes the importance of following safety protocols, wearing appropriate personal protective equipment, and receiving proper training when working with hazardous chemicals.
It is crucial to handle chemicals in a lab setting with care and adhere to safety guidelines to minimize the risk of burns, serious illness, or even death.
Understanding the properties and hazards associated with the chemicals being used, as well as implementing proper safety measures, can greatly reduce the likelihood of accidents or harmful consequences
Learn more about chemicals in the link:
https://brainly.com/question/29886197
#SPJ11
Related Questions
In ionic bonding, atoms _____.
share electrons
are connected by strong electrical forces
lose protons and form ions
stop moving completely
Answers
Answer:
lose protons and form ions
Explanation:
I thjink hope this helps
PLease mark brainliest
Calculate the equivalent weight of 4-toluic acid (CH3C6H4COOH).
Answers
To calculate the equivalent weight of 4-toluic acid (CH3C6H4COOH), you first need to find its molecular weight. The molecular weight is the sum of the atomic weights of all the atoms in the molecule.
For 4-toluic acid:
Carbon (C) = 8 atoms × 12.01 g/mol = 96.08 g/mol
Hydrogen (H) = 8 atoms × 1.01 g/mol = 8.08 g/mol
Oxygen (O) = 2 atoms × 16.00 g/mol = 32.00 g/mol
Now, add the weights together:
96.08 g/mol + 8.08 g/mol + 32.00 g/mol = 136.16 g/mol
The equivalent weight is the molecular weight divided by the number of replaceable hydrogen atoms in the acid, which in the case of 4-toluic acid is 1 (from the -COOH group):
Equivalent weight = 136.16 g/mol / 1 = 136.16 g/mol
So, the equivalent weight of 4-toluic acid is 136.16 g/mol.
Learn more about equivalent weight here:
https://brainly.com/question/1542991
#SPJ11
how many calories are in a dinner containing 3.5x10 ^6 joules
Answers
Answer:
3500000 try this i think it's right not sure
Explanation:
Calories are used to define the unit of energy given by the consumed food in the body. A dinner containing 3.5x10⁶ joules has calories of 836.52 Cal.
What are calories?Calories have been defined as the amount of energy given by the food and its various components, including carbohydrates, proteins, fats, fibers, etc. The stored calories more than the use of those calories results in their storage in the adipose tissues which may lead to obesity.
The food energy from joules can be converted into large calories (Cal) as it is the required amount to raise 1°C of the temperature of a 1 kg substance at 1 atm pressure.
The conversion is done as:
1 J = 0.000239 Cal
3500000 joules = 836.520 Cal
Therefore, the energy of dinner in calories is 836.520 Cal.
Learn more about calories, here:
https://brainly.com/question/22374134
#SPJ2
Why did Chief John Ross decided to sign a treaty with Albert Pike? A. The victory at Wilson’s Creek convinced Ross the Confederacy would win. B. He was working as a spy for the Union. C. He planned to ignore the treaty and move his tribe west. D. Because his tribe needed money to support their interests during the war.
Answers
Answer:
D, i think..
Explanation:
Answer:
It's A.
Explanation:
A.The victory at Wilson's Creek convinced Ross the Confederacy would win.
Carol Dweck's contends that adopting a __________ can help people increase intellectual achievement, promote conflict resolution, reduce aggression, improve willpower, and nurture race relations.
Answers
Carol Dweck contends that adopting a growth mindset can help people increase intellectual achievement, promote conflict resolution, reduce aggression, improve willpower, and nurture race relations. According to Dweck, a growth mindset is the belief that one's abilities and intelligence can be developed through dedication and hard work.
This belief can lead to a desire to learn and improve, as well as a willingness to take on challenges and persevere through obstacles. In the context of intellectual achievement, a growth mindset can lead individuals to embrace challenges, learn from criticism, and persist through difficulties. This can lead to greater success in academic and professional pursuits. In terms of conflict resolution, a growth mindset can foster a willingness to listen to and understand different perspectives, leading to more constructive and productive conversations.
Reducing aggression can also be a result of adopting a growth mindset, as individuals with this mindset tend to focus on self-improvement rather than comparing themselves to others or seeking external validation. This can lead to a greater sense of self-worth and less aggression towards others. Improving willpower is another potential benefit of a growth mindset, as individuals with this mindset tend to view setbacks and challenges as opportunities for growth rather than failures. This can lead to greater resilience and the ability to persevere through difficult situations. Finally, a growth mindset can also nurture race relations by promoting a belief in the potential for change and improvement, both individually and collectively. This can lead to a greater willingness to listen to and understand different perspectives, as well as a greater sense of empathy and compassion towards others.
Learn more about productive conversations here-
https://brainly.com/question/29412775
#SPJ11
choose whether the following target molecule would be better made by reaction with an organolithium reagent or an reagent. In Parts 2 and 3, draw the reagents necessary to prepare this product through two different reactions. 1st attempt Part 1 (1 point) See Periodic Table O See Hint Would this product be made more efficiently by reacting with an organolithium reagent or an organocuprate reagent? ls the reaction you chose an example of 1.2-addition or 1,4 addition? Choose one: O Organolithlum reagent...1.4-addition o Organocuprate reagent.. 1.4-addition Organolithium reagent... 1.2-addition Organocuprate reagent... 1.2-addition
Answers
The given compound is better formed by the reaction of the ketonic compound with alkyl lithium. The organolithium reagent used here is (CH3)₂CHCH₂Li .
What is organolithium ?Organometallic compounds are compounds with one organic carbon -metal bond. These class of compounds are significantly important reagents in synthetic chemistry.
Organolithium compounds has the general formula R-Li. R can be any organic chain. They are used in the alkylation of other organic compounds.
The given initial compound is the benzyl ketone. Where the organolithium group is attaching through 1, 4 addition. The alkyl group is attached with the 4th carbon and the hydrogen from last step hydrolysis is attached to the first carbon of double bond.
Therefore, option a is correct.
Find more on organolithium:
https://brainly.com/question/21262675
#SPJ1
Your complete question is attached below:

NEED HELP Solubility rule

Answers
The solubility rules can be used to deduce that;
1 - Insoluble
2 - Soluble
3 - Soluble
4 - Soluble
5 - Insoluble
6 - Soluble
7 - Soluble
8 - Soluble
9 - Soluble
What does the soluble rule entail?A set of broad recommendations for predicting the solubility of various substances in water are provided by the solubility rule, often known as the solubility guidelines or the solubility table. These principles help determine whether a chemical will dissolve in water to form a homogeneous solution or precipitate out as a solid. They are based on empirical facts.
The designation of the solubility rules served as the basis for labeling the substances as soluble or insoluble.
Learn more about solubility rule:brainly.com/question/4117609
#SPJ1
nitrates, turbidity and acidity. Whichwater sample was likely runoff from a farm?
Answers
Answer:
What is the most likely source of nitrogen in runoff? Fossil fuel emissions.
Grace runs 6 miles in 2 hours. What is her speed?
Answers
What is the importance of sodium chloride
Answers
Answer:
Sodium Chloride is salt
Explanation:
Basically, its used to help absorb and transport nutrients, its also used to help blood pressure along with maintaining the right balance of fluid. These will all be helpful to our bodies and environment.
Answer:
Sodium chloride (NaCl), also known as salt, is an essential compound our body uses to: absorb and transport nutrients. maintain blood pressure. maintain the right balance of fluid.
12.0 moles of li(s) react with 6.0 moles of h3po4 according to the reaction 6 li(s) + 2 h3po4(aq) -> 2 li3po4(aq) + 3 h2(g) what is the theoretical yield (in moles) of h2 gas? (1pts) question 19 - 12.0 moles of li(s) react with 6.0 moles of h3po4 according to the reaction 6 li(s) + 2 h3po4(aq) -> 2 li3po4(aq) + 3 h2(g) what is the theoretical yield (in moles) of h2 gas?
Answers
The theoretical yield (in moles) of H₂ gas produced from the reaction of 12 moles of Li and 6 moles of H₃PO₄ is 6 moles
Balanced equationThe balanced equation for the reaction is given below:
6Li(s) + 2H₃PO₄(aq) -> 2Li₃PO₄(aq) + 3H₂(g)
From the balanced equation above,
6 moles of Li reacted with 2 moles of H₃PO₄.
Next, we shall the limiting reactant. This can be obtained as follow:
From the balanced equation above,
6 moles of Li reacted with 2 moles of H₃PO₄.
Therefore,
12 moles of Li will react with = (12 × 2) / 6 = 4 moles of H₃PO₄
From the above calculation, only 4 moles of H₃PO₄ reacted completely with 12 moles of Li.
Thus, Li is the limiting reactant.
How do I determine the theoretical yield of H₂?The theoretical yield of H₂ can be obtained as follow:
From the balanced equation above,
6 moles of Li reacted to produced 3 moles of H₂.
Therefore,
12 moles of Li will react to produce = (12 × 3) / 6 = 6 moles of H₂
Conclusion: The theoretical yield of H₂ is 6 moles
Learn more about stoichiometry:
https://brainly.com/question/13375719
#SPJ1
An 85.0 kg patient being treated for a serious infection is to receive an iv infusion of 1 /mg kg gentamicin, a powerful antibiotic. the pharmacy has prepared a 250. ml iv bag of normal saline in which 0.500 g of gentamicin has been dissolved. what is the total volume of the iv solution that should be given to the patient? round your answer to the nearest ml
Answers
Answer: 1/ms=8mk
Explanation:
A sealed container at 25oC contains a gas at a pressure of 104 kPa. What is the pressure of the gas when it is heated to 225oC?
1. 304 kPa
2. 936 kPa
3. 174 kPa
4. 62.2 kPa
Answers
Answer:
174 kPa
Explanation:
Given that,
Initial temperature, T₁ = 25° C = 25+273 = 298 K
Final temperature, T₂ = 225°C = 225 + 273 = 498 K
Initial pressure, P₁ = 104 kPa
We need to find the new pressure. The relation between the temperature and pressure is given by :
\(\dfrac{P_1}{T_1}=\dfrac{P_2}{T_2}\)
So,
\(P_2=\dfrac{P_1T_2}{T_1}\\\\P_2=\dfrac{104\times 498}{298}\\\\P_2=173.79\ kPa\)
or
P₂ = 174 kPa
So, the new pressure is 174 kPa.
What happens to the price of the mineral that was extracted when mining companies are required to reclaim the land that they used for mining? Please help me.
Does it go down
Increase
No effect
Or stays the same
Answers
Answer:
innerdisactive react to be step counter function.
Explanation:
i feel
At a constant pressure, a 10.0 L volume of gas is cooled from 546 K to 273 K. What will be the final volume of this gas, assuming no liquefying occurs?
Answers
Answer:
5.0 L
Explanation:
We must recall that pressure is only held constant when referring to Charles law. We then put down the parameters given in the question.
Initial volume V1= 10.0 L
Initial temperature T1= 546 K
Final temperature T2= 273 K
Final volume V2= the unknown
From the statement of Charles law;
V1/T1 = V2/T2
V1T2= V2T1
V2= V1T2/T1
V2= 10.0 × 273/ 546
V2= 5.0 L
Therefore, the new (final) volume is 5.0 L
Answer:
Since the temperature is halved, the volume will also be halved.
The final volume is 5.0 L
Explanation:
Step 1: Data given
The pressure is constant
The initial volume of the gas = 10.0 L
The initial temperature = 546 K
The temperature is cooled to 273 K
Step 2: Calculate the final volume
V1/T1 = V2/T2
⇒with V1 = the initial volume = 10.0L
⇒with T1 = the initial temperature = 546 K
⇒with V2 = the final volume = TO BE DETERMINED
⇒with T2 = the final temperature = 273 K
10.0 L / 546 K = V2 / 273 K
V2 = (10.0 L * 273 K) / 546 K
V2 = 5.0 L
Since the temperature is halved, the volume will also be halved.
The final volume is 5.0 L
1) What is the value of G at 120.0 K for a reaction in which H = +35 kJ/mol and S = 1.50
kJ/(mol.K)?
Answers
Answer:
ΔG = -145kJ/mol
Explanation:
To solve this question we must use the change in Gibbs free energy:
ΔG = ΔH - TΔS
Where ΔG is change in Gibbs free energy = ?
ΔH is enthalpy change = +35kJ/mol
T is absolute temperature = 120.0K
And ΔS is change in entropy = 1.50kJ/molK
Replacing:
ΔG = 35kJ/mol - 120.0K*1.50kJ/molK
ΔG = 35kJ/mol - 180kJ/mol
ΔG = -145kJ/molHow many milliliters of sodium metal, with a density of 0.97 g/mL, would be needed to produce 34.5 grams of sodium hydroxide in the single replacement reaction below?
Unbalanced equation: Na + H2O ---> NaOH + H2
Answers
Volume of Sodium metal : 20.454 ml
Further explanationReaction(balanced) :
2Na + 2H₂O → 2NaOH + H₂
mass NaOH = 34.5 g
mol NaOH(MW=40 g/mol) :
\(\tt mol=\dfrac{mass}{MW}\\\\mol=\dfrac{34.5}{40}\\\\mol=0.8625\)
From the equation, mol ratio of Na : NaOH = 2 : 2, so mol Na=mol NaOH=0.8625
Mass Na (Ar=23 g/mol):
\(\tt mass=mol\times MW\\\\mass=0.8625\times 23\\\\mass=19.84~g\)
Volume Na :
\(\tt V=\dfrac{m}{\rho}\\\\V=\dfrac{19.84~g}{0.97~g/ml}\\\\V=20.454~ml\)
Use Lewis symbols to show how MgCl2 will be formed from Mg and Cl2.
Answers
This is a type of bonding that is formed from the from the attraction of oppositely charged ions in a compound.
For instance, MgCl2 is an ionic compound because the 2 positive ions wipossessed by the magnessium atom will attract each of the negtaive ion possessed by each of the chlorine atom to form the magnessium chloride compound
Using the Lewis symbol to demonstrate the bondng:
From the disgram, the negative ions on chlorine atoms will get attracted to the positive ions on the magnessium ion.
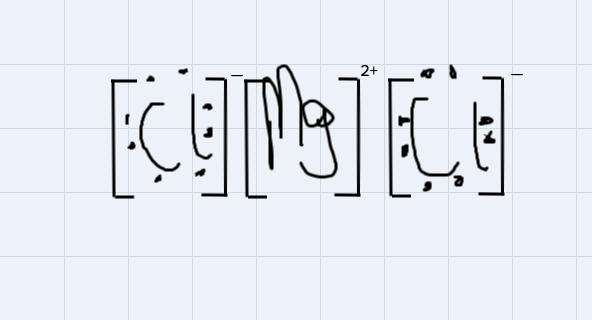
A 1.83 mol sample of carbon reacts with excess zinc oxide to produce zinc and carbon dioxide. How many moles of zinc oxide are consumed?
Answers
Answer:
3.66 moles of Zinc Oxide are consumed
Explanation:
Based on the reaction:
C + 2ZnO → 2Zn + CO₂
Where 1 mole of Carbon reacts with 2 moles of Zin Oxide to produce 2 moles of Zinc and 1 mole of Carbon Dioxide.
To solve this question, we must convert the moles of carbon added to the moles of Zinc Oxide that react using the chemical equation (1mol C = 2mol ZnO):
1.83 moles C * (2mol ZnO / 1mol C) =
3.66 moles of Zinc Oxide are consumed
Using the mole ratios determine how many moles of KClO3 must be decomposed whe
5.00g of O₂ is to be produced. Round your answer to four decimal places.
Mole ratio for
O2 to KCIO3
Is 2:1
Answers
To find the number of moles of KClO₃ that must be decomposed when 5.00g of O₂ is produced, we need to set up a proportion using the molar mass of KClO₃.
The molar mass of KClO₃ can be calculated as follows:
K (39.10 g/mol) + Cl (35.45 g/mol) + O₃ (16.00 g/mol × 3) = 122.55 g/mol
Now, set up the proportion:
2 moles O₂ / 1 mole KClO₃ = 5.00 g O₂ / x moles KClO₃
Cross-multiply and solve for x:
2 moles O₂ × x moles KClO₃ = 5.00 g O₂ × 1 mole KClO₃
2x = 5.00 g O₂ × 1 mole KClO₃
2x = 5.00 g O₂ / 122.55 g/mol
2x = 0.0408 mol O₂
Now, solve for x:
x = 0.0408 mol O₂ / 2
x = 0.0204 mol KClO₃
Therefore, 0.0204 moles of KClO₃ must be decomposed when 5.00g of O₂ is produced.
To what temperature must a given Mass of nitrogen at 0°c heated so that both it volume and pressure will be double.
Answers
1/4 temperature must give Mass of nitrogen at 0°c heated so that both it volume and pressure will be double.
To double both the volume and pressure of a given mass of nitrogen at 0°C, we can utilize the combined gas law, which relates the initial and final states of a gas. The combined gas law is expressed as:
(P1 * V1) / T1 = (P2 * V2) / T2
Where P1 and P2 are the initial and final pressures, V1 and V2 are the initial and final volumes, and T1 and T2 are the initial and final temperatures.
Since we want to double both the volume and pressure, we can set P2 = 2P1 and V2 = 2V1. Plugging these values into the combined gas law equation, we get:
(2P1 * 2V1) / T1 = P1 * V1 / T2
Simplifying the equation, we find:
4P1V1 = P1V1 / T2
Cancelling out the common terms, we have:
4 = 1 / T2
Rearranging the equation, we find:
T2 = 1 / 4
Therefore, to double both the volume and pressure of the given mass of nitrogen at 0°C, it must be heated to a temperature of 1/4 or 0.25 times its initial temperature.
Know more about combined gas law here:
https://brainly.com/question/13154969
#SPJ8
a mixture of gaseous hydrogen and nitrogen is considered pure substance Select one: True False
Answers
False. A mixture of gaseous hydrogen and nitrogen cannot be considered a pure substance because it contains two or more different types of particles (hydrogen and nitrogen) which cannot be broken down by physical means into simpler substances of different components.
In chemistry, a pure substance is a type of matter consisting of a single type of particle or matter. It can be broken down into simpler substances, either chemically or physically, but it always contains the same number of atoms or molecules and therefore retains its chemical properties.
Pure substances include elements (such as oxygen, iron, and gold) and compounds (such as water, carbon dioxide, and sodium chloride). Mixtures, on the other hand, are composed of two or more pure substances and can be separated into their components by physical means, such as filtration or distillation.
Therefore, a mixture of gaseous hydrogen and nitrogen is not a pure substance.
To know more about considered visit :
https://brainly.com/question/30746025
#SPJ11
Sodium carbonate is a reagent that may be used to standardize acids in the same way you used KHP in
this experiment. In such a standardization, it was found that a 0.512 g sample of sodium carbonate
required 26.30 mL of a sulfuric acid solution to reach the end point for the reaction.
Na2CO3(aq) + H2SO4(aq) -> H2O(1)+ CO2(g) + Na2SO4(aq)
Answers
sodium carbonate can be used to standardize acids in a similar manner as KHP. In this specific standardization, a 0.512 g sample of sodium carbonate required 26.30 mL of sulfuric acid solution to reach the end point for the reaction.
The reaction between sodium carbonate and sulfuric acid results in the formation of water, carbon dioxide, and sodium sulfate. The balanced chemical equation for this reaction is Na2CO3(aq) + H2SO4(aq) -> H2O(1)+ CO2(g) + Na2SO4(aq). By adding a known amount of sodium carbonate to a standardized acid solution, the amount of acid required to neutralize the base can be determined. This information can then be used to calculate the concentration of the acid solution.
To calculate the concentration of the sulfuric acid solution, follow these steps:
1. Determine the moles of sodium carbonate (Na2CO3) in the 0.512 g sample:
Molar mass of Na2CO3 = (2 * 22.99) + 12.01 + (3 * 16) = 105.99 g/mol
Moles of Na2CO3 = 0.512 g / 105.99 g/mol = 0.00483 mol
2. Identify the balanced chemical equation:
Na2CO3(aq) + H2SO4(aq) -> H2O(l) + CO2(g) + Na2SO4(aq).
To know more about sodium carbonate visit :
https://brainly.com/question/31344166
#SPJ11
Which of these describes a challenge that the engineer of a high-altitude
airplane would need to consider?
A. More fuel is used at higher altitudes.
B. The airplane may succumb to the power of gravity as lift
decreases at higher altitudes.
C. The airplane may need a heat shield for protection from sonic
booms.
O D. The airplane will create more drag at higher altitudes than lower
altitudes.
Answers
Answer:
I think its (B)
Explanation:
as when the air thins out at higher elevations, your lift decreases so at some point, the plane cant produce enough lift to overcome gravity.
2
How many molecules are in 2.47 moles of CC14? *
Answers
1.49x10^24 molecules
What is the difference between nuclear mass and atomic mass?.
Answers
which equilibrium reaction will experience a shift towards the products in equilibrium position when the concentration of ni2 is increased?
Answers
Reaction Ni²⁺(aq) + 6NH₃(aq) ⇌ [Ni(NH₃)₆]²⁺ will experience a shift towards the products in equilibrium position when the concentration of Ni²⁺ is increased.
What is equilibrium shift?According to Le Chatelier's Principle: When the equilibrium is stressed, the response changes to relieve the stress. This means that adding a reactant moves the equilibrium away from the reactant to the right. Adding a product moves the equilibrium away from the product and to the left.
Equilibrium type:
Stable equilibrium.Unstable equilibrium.Neutral equilibrium.As the concentration of Ni²⁺(aq) increases, the system attempts to reach equilibrium by reducing the increased coefficient according to Le Chatelier's principle. To do this, the concentration of Ni²⁺(aq) must be lowered. Therefore, it is necessary to lower the Ni²+(aq) concentration by promoting forward reaction.
To know more about Chatelier's Principle visit:
https://brainly.com/question/17371799
#SPJ4
The given question was incomplete so the complete question is as follows:
Which equilibrium reaction will experience a shift towards the products in equilibrium position when the concentration of Ni2+ is increased? View Available Hint(s) Which equilibrium reaction will experience a shift towards the products in equilibrium position when the concentration of is increased?
Ni(OH)₂(s)⇌Ni²⁺(aq)+2OH⁻(aq)
[Ni(H2O)₆]²⁺(aq)+3En(aq)⇌[Ni(En)₃]²⁺(aq)+6NH₃(aq)
Ni²⁺(aq)+6NH₃(aq)⇌[Ni(NH₃)₆]²⁺
NiS(s)⇌Ni²⁺(aq)+S²⁻(aq)
Give another example of electrical energy being transferred into light
Answers
Answer: lightbulb, lamp, nightlight
Explanation:
the bohr model was based on the helium atom and rings around the nucleus are called orbits. true or false
Answers
The statement "the Bohr model was based on the helium atom and rings around the nucleus are called orbits" is partially true and partially false.
The Bohr model was actually based on the hydrogen atom, not the helium atom. However, it is true that the rings around the nucleus are called orbits. So, the correct answer would be that the statement is both true and false due to the inaccuracy regarding the atom the model was based on.
The statement mentioned in the question is not entirely accurate. The Bohr model was based on the hydrogen atom, not the helium atom. The Bohr model was developed to explain the hydrogen atom's atomic structure, which is simpler than the helium atom's atomic structure. The hydrogen atom has one electron and one proton, whereas the helium atom has two electrons and two protons.
On the other hand, the second part of the statement is accurate. The rings around the nucleus in the Bohr model are called orbits. These orbits are specific, quantized energy levels that electrons can occupy in an atom. When an electron transitions between these energy levels, it emits or absorbs a photon of specific energy, which gives rise to the spectral lines observed in atomic spectra.
Therefore, the statement "the Bohr model was based on the helium atom and rings around the nucleus are called orbits" is partially true and partially false. The first part of the statement is incorrect because the Bohr model was based on the hydrogen atom, not the helium atom. The second part of the statement is accurate because the rings around the nucleus in the Bohr model are called orbits.
To know more about "Bohr model " refer here:
https://brainly.com/question/13606024#
#SPJ11
a 25g sample of an unknown gas has a volume of 3.9 l at 25 celsius and 2 atm pressure. it is found to be 7.7% hydrogen and 92.3% carbon. what is its molecular formula
Answers
The molecular formula of the given sample is obtained by the ideal gas equation that is PV=nRT.
here volume given is 3.9L and pressure give n is 2 atm and temperature given is 25 C, amount of the sample given is 25 g. here we put the expression of , PV= nRT .then it is found to be 7.7% hydrogen and 92.3% carbon. first we should convert the percentage in to mass. so,7.7% becomes 7.7g and 92.3% become 92.3 gram. Then transform the mass into moles. so H becomes 7.7/1.01= 7.62 mole. and C becomes, 92.3/12.01= 7.69 moles. Then put all these in the relation of empirical formula and molecular formula. Then the molecular formula of the unknown gas can be obtained.
To learn more about Ideal gas equation please visit:
https://brainly.com/question/13248885
#SPJ4