Chlorine has two naturally occurring isotopes, 35cl and 37cl. What is the mass number of each? how many protons, neutrons, and electrons are present in each?.
Answers
The mass number \(_{17} Cl^{35}\) is 35.
The mass number \(_{17} Cl^{37}\) is 37.
isotopes of \(_{17} Cl^{35}\) contains. and isotopes \(_{17} Cl^{37}\) contain.
protons=17 protons= 17
neutrons= 18 neutrons=20
electrons= 17 electrons=17
Define Isotopes
Isotopes are two or more atom types that share the same atomic number (number of protons in their nuclei) and position in the periodic table (and, therefore, belong to the same chemical element). However, isotopes have different nucleon numbers (mass numbers) because they have varying numbers of neutrons in their nuclei. All isotopes of a given element have nearly identical chemical properties but differ in terms of atomic mass and physical characteristics.
Learn more about Isotopes here:-
https://brainly.com/question/24337473
#SPJ4
Related Questions
HELP ME ASAP!
what is the purpose of putting cabbage water, baking soda, and vinegar experinment?
Answers
test for different types of molecules called acids and bases.
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
Potassium hydroxide, also known as lye, dissociates into metal and hydroxide ions in water.
The resulting solution is caustic and used for industrial purposes. What describes the dissociated solution?
O Weak acid
O Strong acid
O Strong base
O Weak base
Answers
Answer:
strong acid and weak base
Explanation:
#carryonlearning
In the given question dissociated solution describes the property of the strong base.
What are bases?Bases are substances which gives hydroxide ion into the solution.
Dissociation reaction of caustic soda will be represented as:
KOH → K⁺ + OH⁻
From the above dissociation we conclude that potassium hydroxide is fully dissociates into their ions means it is a strong base and will make the solution caustic.
Weak acid & strong acid is wrong because acids gives H⁺ ion not OH⁻.Weak base is also wrong as weak bases show partial dissociation only.Hence potassium hydroxide is a strong base.
To know more about strong bases, visit the below link:
https://brainly.com/question/7245674
#SPJ2
which metal oxides can react with carbonmonoxide in the same way as zinc oxide
Answers
Answer:The reaction of zinc oxide with carbon monoxide forms the product zinc metal and carbon dioxide. Zinc oxide is reduced to metallic zinc and carbon monoxide is oxidized to carbon dioxide.
Explanation:
Calculate the molality of a solution prepared by dissolving 13.3 g of kcl in 750.0 ml of water.
Answers
The molality of a solution prepared by dissolving 13.3 g of kcl in 750.0 ml of water is 0.23 moles per kilogram.
What is molality?
The molality of a solution is the number of moles of solute present in per kilogram of the solvent is called the molality of the solution and cannot be affected by temperature.
Molality = weight / mass × 1000/ volume if water in ml.
mass of KCl = 74.55
substituting the value in the formula,
molality = 13.3 g / 74.55 × 1000/ 750
molality = 0.23 moles per kilogram.
Therefore, 0.23 moles per kilogram is the molality of a solution prepared by dissolving 13.3 g of KCL in 750.0 ml of water.
Learn more about molality, here:
https://brainly.com/question/26921570
#SPJ4
The pH of a solution can be determined using the formula pH=−log[H
+
], where H
+
is the hydrogen ion concentration in the solution. a. The hydrogen ion concentration of a particular brand of fruit juice is 0.0003 mol/L. Determine the pH of the solution, to the nearest tenth. ( 1 mark) b. A tomato has a pH of 3.0. Algebraically determine the hydrogen ion concentration of this solution. (2 marks)
Answers
(a)The pH of the fruit juice solution is approximately 3.5. (b) The hydrogen ion concentration of the tomato solution is 0.001 mol/L.
(b)The hydrogen ion concentration of the tomato solution is 0.001 mol/L.
(a). The hydrogen ion concentration of the fruit juice is 0.0003 mol/L. We can determine the pH of the solution using the formula pH = -log[H⁺].
pH = -log(0.0003)
pH ≈ -log(3 × 10⁻⁴)
Using a calculator, we can calculate the logarithm:
pH ≈ -(-3.5229) (rounded to the nearest tenth)
pH ≈ 3.5
Therefore, the pH of the fruit juice solution is approximately 3.5.
(b). A tomato has a pH of 3.0. We can determine the hydrogen ion concentration of this solution by rearranging the formula pH = -log[H⁺] to solve for [H⁺].
[H⁺] = 10(-pH)
[H⁺] = 10⁻³
[H⁺] = 0.001 mol/L
Therefore, the hydrogen ion concentration of the tomato solution is 0.001 mol/L.
To know more about hydrogen ion:
https://brainly.com/question/24673381
#SPJ4
A chemist wants to extract copper metal from copper chloride solution. The chemist places 1. 50 grams of aluminum foil in a solution of 14 grams of copper (II) chloride. A single replacement reaction takes place. What best explains the state of the reaction mixture after the reaction?
Unbalanced equation: CuCl2 + Al → AlCl3 + Cu
Less than 6. 0 grams of copper is formed, and some aluminum is left in the reaction mixture.
More than 6. 5 grams of copper is formed, and some aluminum is left in the reaction mixture.
Less than 6. 0 grams of copper is formed, and some copper chloride is left in the reaction mixture.
More than 6. 5 grams of copper is formed, and some copper chloride is left in the reaction mixture
Answers
Less than 6. 0 grams of copper i.e. 0.36 g is formed, and some aluminum is left behind in the reaction ingredients.
The amount of copper made is calculated from the balanced equation of the reaction.
The balanced equation :
3 CuCl2 + 2 Al → 2 AlCl3 + 3 Cu
molar mass of CuCl₂ = 132 g/mol
molar mass of Al = 27.0 g
molar mass of Cu = 64.0 g
moles of aluminium = 0.50 / 27 = 0.018 moles
moles of CuCl₂ produced = 0.75/132 = 0.00568 moles
So, CuCl₂ is acting as the limiting reagent in the reaction.
3 moles of CuCl₂ makes 3 moles of Cu
Similarly,
0.00568 moles CuCl₂ will make 0.00568 moles of Cu
Mass of Cu produced = 0.00568 * 64 = 0.36 g
So, Less than 6. 0 grams of copper is formed, and some aluminum is left in the reaction mixture as CuCl2 acts as limiting reagent.
Learn more about Copper
brainly.com/question/13677872
#SPJ4
Write two reactions showing nascent Hydrogen is more reactive than
molecular Hydrogen.
Answers
Answer:
1.) Nascent Hydrogen has more energy than Molecular Hydrogen.
2.) Nascent Hydrogen is are more on the side of atomic and since atoms are more active than molecules, Nascent Hydrogen becomes more reactive.
the mass of carbon, nitrogen, and hydrogen are 12 amu, 16 amu, and 1 amu. what is the molecular weight of this molecule?
Answers
To calculate the molecular weight of this molecule, However, we can tell you that the sum of the atomic weights of the elements in a molecule is called its molecular weight.
In this case, the molecular weight would be the sum of the atomic weights of carbon, nitrogen, and hydrogen in the molecule.The correct amu for carbon, nitrogen, and hydrogen are 12 amu, 14 amu, and 1 amu, respectively.
To find the molecular weight of a molecule that includes one atom of each element, simply add their respective amu values together: 12 amu (carbon) + 14 amu (nitrogen) + 1 amu (hydrogen) = 27 amu. The molecular weight of this molecule is 27 amu.
To know more about hydrogen click here
brainly.com/question/30037191
#SPJ11
To determine the molecular weight of the molecule, we need to know the formula of the molecule. However, based on the information given, we can make an educated guess that the molecule is likely to be a combination of carbon, nitrogen, and hydrogen atoms.
Assuming that the molecule has the formula CHN, we can calculate its molecular weight as follows:
Molecular weight of CHN = (mass of carbon x number of carbon atoms) + (mass of hydrogen x number of hydrogen atoms) + (mass of nitrogen x number of nitrogen atoms)
= (12 amu x 1) + (1 amu x 1) + (16 amu x 1)
= 12 amu + 1 amu + 16 amu
= 29 amu
Therefore, the molecular weight of the molecule with carbon, nitrogen, and hydrogen atoms having masses of 12 amu, 16 amu, and 1 amu, respectively, is 29 amu.
To know more about Molecular weight of CHN:
https://brainly.com/question/28297551
#SPJ11
Which of the following statements below is true?
A.)Measurement of a physical property does change the identity of the substance
B.)Measurement of a chemical property does not change the identity of the
substance
C.)Measurement of a physical property can only be measured when the identity of
the substance changes
D.)Measurement of a chemical property occurs as the identity of the substance
changes
Answers
The correct statements from the given statement is that the measurement of a chemical property occurs as the identity of the substance changes.
Chemical change is defined as a change is occurred when the substance is transformed into one or more substances. This process is known as a chemical reaction. Chemical property is the characteristic of a particular substance which is observed in a chemical reaction. Some examples of chemical properties are flammability, change in pH, heat of combustion, acidity. For example, when an iron metal comes into contact of air and moisture, it is converted into a rust, which has a chemical formula Fe₂O₃.nH₂O.
Therefore, the measurement of a chemical property occurs as the identity of the substance changes. Hence, 'option d' is correct.
To know more about chemical reaction
https://brainly.com/question/28776833
#SPJ1
A 2.0 L container of nitrogen gas had a pressure of 3.2 atm. What volume would be necessary to decrease the pressure to 1.0 atm
Answers
Answer:
6.4 L
Explanation:
When all other variables are held constant, you can use Boyle's Law to find the missing volume:
P₁V₁ = P₂V₂
In this equation, "P₁" and "V₁" represent the initial pressure and volume. "P₂" and "V₂" represent the final pressure and volume. You can find the theoretical volume by plugging the given values into the equation and simplifying.
P₁ = 3.2 atm P₂ = 1.0 atm
V₁ = 2.0 L V₂ = ? L
P₁V₁ = P₂V₂ <----- Boyle's Law
(3.2 atm)(2.0 L) = (1.0 atm)V₂ <----- Insert values
6.4 = (1.0 atm)V₂ <----- Simplify left side
6.4 = V₂ <----- Divide both sides by 1.0
Answer:
6.4L or C on Brainly.
Explanation:
what is the ionic charge lf this atom
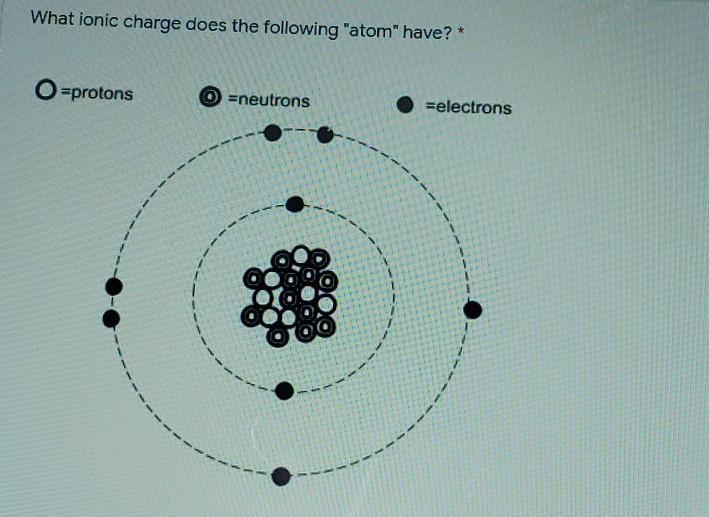
Answers
Answer:
An ionic charge of 2-
Explanation:
The number of electrons is 8 and the number of protons is 2, subtract the two and the difference is the ionic charge. Hope this helps! :)
Answer:
This answer would be 2
Explanation:
The way that I have always done it is you put protons in tally marks (without crossing) over the top of the neutrons and mark them out one by one and whichever one that you have leftover that is your charge.
Hope this helps!
why does spoiled food produce foul smell
Answers
Answer:
There is spoilage microbes growing on the food, such as bacteria, yeasts, and mold. The odor can either come from the chemicals that are released from the food as the microbes decompose it down, or the chemicals are produced by the microbes themselves.
Answer:
When food goes bad and starts to become pungent, it is most often due to the growth of spoilage microbes such as bacteria, yeasts and mold. Odors can come from two sources: chemicals that are released from the food as the microbes decompose it, or chemicals produced directly by the microbes themselves.
Hope this answer correct :)
A sample of gaseous arsine (AsH3) in a 460 mL flask at 332 Torr and 223 K, is heated to 437 K, at which temperature arsine decom- poses to solid arsenic and hydrogen gas. The flask is then cooled to 273 K, at which tem- perature the pressure in the flask is 488 Torr. What percentage of arsine molecules have de- composed?
Answer in units of %.
Answers
Answer:
28/95 = 29.,5 % of Arsine decomposed
Explanation:A sample of gaseous arsine (AsH3) in a 460 mL flask at 332 Torr and 223 K, is heated to 437 K, at which temperature arsine decom- poses to solid arsenic and hydrogen gas. The flask is then cooled to 273 K, at which tem- perature the pressure in the flask is 488 Torr. What percentage of arsine molecules have de- composed?
Answer in units of %.
initial pressure 332 Torr initial volume 0.46 L initial temperature 223K
final pressure 488 Torr final volume 0.46 L final 273 K
Torr is 1/760 atm 332 torr = 0.437 atm 488 Torr =0.642 atm
PV = nRT so n=RT/PV
INITIAL n= 0.082 X 223/(0.437)(0.46) = 91 moles
final n= 0.082 X 273 / (.437)(488) = 105 moles
2AsH3----------> 2As + 3H2
x moles of Arsine decomposed to make 1.5 moles of H2
the final number of moles was
(91 -X)+ 1.5 X = 105 moles
91 + 0.5 X = 105
0.5 X = 14
X =28
CHECK
if 28 moles of Arsine , then the container would have
91 --28 + 1.5(28) = 91 +14 =105 check
so 28/95 = 29.,5 % of Arsine decomposed
Your answer
(quit)
polyalchemVirtuoso
Answer:
Explanation:
ADD YOUR ANSWER
Ask maryhobbs about this question...
New questions in Chemistry
in what area of the united states do the fewest people live
Read each step. Then put the steps in order from first to last. Write 1 for the first step, 2 for the second step and so on. 1. Fossils fuels are burn…
When unbalanced forces act on an object that is in motion, the object can: A. change speed, direction, or both. B. only change direction. C. only s…
What is the missing letter in H,J,K,L,M,N,O,P.? And what is the opposite of HATE.? And what is the opposite of ME.? Think about it -,-
what type of reaction is Fe(NO3)3 + 3LiOH = Fe(OH)3 + 3LiNO3
Please answer this quickly. the answers that you find online are not correct. I will mark brainliest if you get it right. NO SPAM!!!!For the reaction …
cuantos gramos de soluto son necesario para preparar 50 ml de Ca(NO3)2 al 4% (w/v)
Calculate the moles of O in 0.182 mole C6H14O. Calculate the number of H atoms in 0.182 mole C6H14O. Calculate the number of C atoms in 0.182 mole C…
When a sulfur atom and an oxygen atom bond to produce substance C, electrons are? a. delocalised b. shared c. transferred
What is oxidation state?
LOAD MORE
Previous
Next
Ask
From the values of Delta H and Delta S predict which of the following reactions would be spontaneous at 25degree C. Calculate the minimum temperature at which each reaction will become spontaneous. Enter "NONE" if the reaction is not spontaneous at any temperature. (a) Delta H = 12.6 kJ/mol, Delta S = 93 J/K middot mol spontaneous at 25degree C not spontaneous at 25degree C (b) Delta H = 9.5 kJ/mol, Delta S = -94.0 J/K middot mol spontaneous at 25degree C not spontaneous at 25degree C
Answers
(a) The reaction with Delta H = 12.6 kJ/mol and Delta S = 93 J/K·mol is spontaneous at 25°C.
(b) The reaction with Delta H = 9.5 kJ/mol and Delta S = -94.0 J/K·mol is not spontaneous at 25°C.
To determine whether a reaction is spontaneous at a given temperature, we can use the Gibbs free energy equation: Delta G = Delta H - T·Delta S, where Delta G is the change in Gibbs free energy, Delta H is the change in enthalpy, Delta S is the change in entropy, and T is the temperature in Kelvin.
For a reaction to be spontaneous, Delta G must be negative. If Delta H is negative (exothermic) and Delta S is positive (increase in disorder), the reaction is more likely to be spontaneous.
(a) For the reaction with Delta H = 12.6 kJ/mol and Delta S = 93 J/K·mol, we have Delta G = 12.6 kJ/mol - (25 + 273) K·(93 J/K·mol/1000 J/kJ) = -5.25 kJ/mol. Since Delta G is negative, the reaction is spontaneous at 25°C.
(b) For the reaction with Delta H = 9.5 kJ/mol and Delta S = -94.0 J/K·mol, we have Delta G = 9.5 kJ/mol - (25 + 273) K·(-94.0 J/K·mol/1000 J/kJ) = 3.57 kJ/mol. Since Delta G is positive, the reaction is not spontaneous at 25°C.
To determine the minimum temperature at which a non-spontaneous reaction becomes spontaneous, we can set Delta G equal to zero and solve for T in the equation Delta G = Delta H - T·Delta S. However, in this case, both reactions are either spontaneous or non-spontaneous at 25°C, so we do not need to calculate the minimum temperature.
To know more about Gibbs free energy refer here:
https://brainly.com/question/29753420#
#SPJ11
Somebody please help me with this.

Answers
Answer:
2. Chemical reactions changes occur when the bonds between atoms in a molecule are created or destroyed.
3. When you reach your its boiling point, the molecules in liquid have enough energy to become a gas.
4. When you have a solid with more than one type of compound, it is called a mixture.
5. A Crystal is a form of solid where the atoms are arranged is a very specific order.
6. If you have different types of molecules dissolved in a liquid, it is called a solution
Why does water boil at 100°C and methane boil at -161°C
Please help me
Answers
Explanation:
The main difference in boiling points are due to intermolecular attractions. With water, it is a polar molecule, has hydrogen bonding, and exhibits london forces. All of these combined, make H2O bonds very strong, which means more energy is required to transition from one state to another, leading to higher boiling points.
On the other hand, methane is a non-polar molecule that only exhibits very weak london forces. It being a non-polar molecule, means its bonds are very weak, which means less energy is required to boil methane.
Also, the Celsius system was made so that water would be a reference. This is a reason for why its boiling point temperature is convenient
How many moles are in 2. 5 g of N2? 0. 089 moles 0. 18 moles 1. 3 moles 11 moles.
Answers
Answer:
moles = mass divided by molar mass
Molar mass of Nitrogen gas (N2) = 14*2 = 28 g/mol
Mass of Nitrogen gas = 5g
Moles = 5/28
Moles = 0.18 (rounded)
How do the products of chemical reactions compare to their reactants? Question 7 options: The products usually weigh more than the reactants. The products often have completely different properties than the reactants. The products usually have more atoms than the reactants. The products are usually more toxic than the reactants
Answers
Answer:
The second choice.
Explanation:
The products often have completely different properties than the reactants.
The first and third are wrong because matter is always preserved in a chemical reaction.
The last is not true.
An analytical chemist is involved with
Answers
Answer:
basic laboratory research, perform process and product development
Explanation:
good luck <3
Question 28 (4 points)
Complete the following table:
Substance
pH
POH
[H] M
A
3.45
B
0.26
For the concentration fill in the blank, I used a decimal with a leading zero and two sig figs.
Answers
Answer:
Pls type your question in another manner cos I really cant decipher what you wrote the way you typed this pls
Gene Linkage, Recombination, and Independent Assortment · Patterns of Inheritance with Gel Electrophoresis Analysis · Simple Genetics Simulation. Metabolism.
Answers
In terms of genetics and genomics, linkage describes how closely genes or other DNA sequences are located to one another on the same chromosome. The likelihood that two genes or sequences on a chromosome will be inherited together increases with their proximity.
When two genes are adjacent to one another on the same chromosome, they are considered to be linked because they do not assort separately. Linked genes have a recombination frequency of less than 50%, whereas genes on distinct chromosomes assort independently and have a 50% recombination frequency.
According to Mendel's law of independent assortment, the alleles of two or more distinct genes are independently selected into gametes. To put it another way, a gamete's allele for one gene does not affect the allele it obtains for another gene.
For single-gene disorders, there are basically five different ways that inheritance can occur: autosomal dominant, autosomal recessive, X-linked dominant, X-linked recessive, and mitochondrial. Both simple multi-factorial diseases and complex single-gene diseases frequently exhibit genetic variability.
The user can track the frequency changes of two alleles over time using this simulation. While changes signify evolution, a population that does not change over time is said to be in genetic equilibrium. This simulation can be used to examine genetic drift, natural selection, and mutation.
Learn more about genetic equilibrium here brainly.com/question/1286980
#SPJ4
what should you do if you are wearing a tie in lab day? what do you think your teacher will suggest other than removing the tie?
Answers
Answer: Probably why are you wearing it and why do you have it on
Explanation:
Answer:
remove it, tuck it under ur shirt
Explanation:
Question 17 of 25
Which of the following is the energy of motion?
A. Nuclear energy
B. Chemical potential energy
C. Kinetic energy
D. Gravitational potential energy
SUOMI
Answers
Answer:
c. kinetic energy
Explanation:
Which of the following flows is not driven by pressure differences?
1: A deep breath
2: A river
3: A gust of wind
4: A sip through a drinking straw
Answers
Answer:
I think it is option (b) river
The river flows is not driven by pressure differences.
The wind often blows due to of differences in air pressure from one location to another. Wind blows often from zones of high pressure toward zones of low pressure and thus when the high pressure point is very close to the low pressure point the wind can blow very fast.But the river is not driven by pressure differences as it flows in its course.Conclusively we can say that the river flows is not driven by pressure differences
Learn more from:
https://brainly.com/question/10152119
14. The atoms of element X contains nineteen electrons. With which of the following elements will the chemistry of Z be similar? a Aluminum b) Bromine c) Lithium d) Magnesium
Answers
First of all, Z is unknown. I hope it is a mistake.
Now, it is given that the element X has nineteen electrons. This proves that X is actually Potassium.
As per the periodic table, both Potassium and Lithium belongs to group 1 as their valency is 1 because of the presence of only one electron in the outermost shell of electrons i.e., they lose an electron during a chemical reaction to form a stable compound. Furthermore, both are metallic.
Magnesium belongs to group 2 and hence its valency is two, which is different from potassium though it is metallic. Similiarly, bromine belongs to group 17 and gains one electron during a reaction in contrast to potassium.
( No internal links available for reference. For clarification, check the periodic table).
Type the correct answer in the box. Express your answer to two significant figures.
A reaction between 1.7 moles of zinc Ipdide and excess sodium carbonate ylelds 12.6 grams of zinc carbonate. This is the equation for the
reaction:
Na2CO3 + Zniz - 2Nal +
ZnCoz.
What is the percent yield of zinc carbonate?
The percent yield of zinc carbonate is
5.91
1X.

Answers
Answer: The percent yield of zinc carbonate is 5.91 %
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}\)
\(\text{Moles of} ZnCO_3=\frac{12.6 g}{125.4g/mol}=0.100moles\)
\(ZnI_2\) is the limiting reagent as it limits the formation of product and \(Na_2CO_3\) is the excess reagent.
\(Na_2CO_3+ZnI_2\rightarrow 2NaI+ZnCO_3\)
According to stoichiometry :
1 mole of produce = 1 mole of
Thus 1.7 moles of \(ZnI_2\) will produce=\(\frac{1}{1}\times 1.7=1.7moles\) of \(ZnCO_3\)
Theoretical yield of \(ZnCO_3=moles\times {\text {Molar mass}}=1.7moles\times 125.4g/mol=213.2g\)
percentage yield = \(\frac{\text {Experimental yield}}{\text {Theoretical yield}}\times 100=\frac{12.6g}{213.2g}\times 100=5.91\%\)
Answer:
the other person was right EXCEPT it says in 2 significant numbers so the answer is 5.9
Explanation:
i hope this helps. have a wonderful day :))

Find the percent of water in Zn3(PO4)2 x 7H2O
Answers
Answer:
24.66%
Explanation:
To find the percent of water (H2O) in the following compound: Zn3(PO4)2. 7H2O
The atomic mass of the elements are as follows:
Zn = 65g/mol
P = 31g/mol
O = 16g/mol
H = 1g/mol
Hence, molar mass of the compound is as follows:
65(3) + {31 + 16(4)} 2 + 7{1(2) + 16}
195 + 95(2) + 7(18)
195 + 190 + 126
511g/mol
Molar mass of 7H2O
= 7(18)
= 126g/mol
Hence, the percent of H2O in the compound is:
Molar Mass of 7H2O/molar mass of compound × 100
= 126/511 × 100
= 0.2466 × 100
= 24.66%
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
How many neutrons does the isotope of lithium have?
A) 8
B) 5
C) 4
D) 3
Answer quick plzzz
Answers
Answer:
B)
Explanation:
it has 5