Did the control experiment verify or refute the results from exercise 1? use your results from exercises 1 and 2 to validate your answer.
Experiment 1 E Data Table 1 B Data Table 2 Data Table 1: Antacid Neutralization Data Mass of 0. 59 Crushed Antacid (9) Concentration 1. 0 of HCI (M) Volume HCI 5. 0 (mL) Concentration 1. 0 of NaOH (M) Initial NaOH 9. 4 mL Volume (mL) Final NaOH 8. 2 mL Volume (mL) Total Volume 1. 2 mL of NaOH Used (mL) Experiment 1 Data Table 1 Data Table 2 Data Table 2: Experimental Results I 0. 1825 g 0. 0012 HCl available for neutralization (g): Moles of NaOH required to reach stoichiometric point (mol): HCI neutralized by antacid (g): НСІ neutralized per gram of antacid (9) 0. 1387 0. 2774 Experiment 2 El Data Table 3 B Data Table 4 Data Table 3: Control Experiment Data Concentration 1. 0 of HCI (M) Volume HCI 5. 0 (mL) Concentration 1. 0 of NaOH (M) Initial NaOH 9. 2 mL Volume (mL) Final NaOH 3. 6 mL Volume (mL) Total Volume 5. 6 mL of NaOH Used (mL) Data Table 4: Control Experiment Results 0. 2049 Moles of 0. 0056mol NaOH needed to neutralize 5. 0 mL of 1. OM HCI (mol): Grams of HCI neutralized (g): NaOH 4. 4mL volume difference between back titration and control (ml): Grams of 0. 160g HCI neutralized by NaOH volume difference (9)
Answers
Volume difference between back titration and controlHCI neutralized by NaOH volume difference is 0.0056 moles.
Given,
* Mass of Antacid = 0.5g
* [HCl] = 1M
* \(V_{HCl}\) = 5 ml (pipetted out)
* [NaOH] = 1M
* \(V_{NaOH}\) - 1.2 ml (consumed)
* Amt. of HCl avaliable for neutralisation = 0.1825 g
* No. of moles of NaOH req. to reach eq. point = 0.0012
* Amt. of HCl neutralised by antalid = 0.1387 g
* Amt. of HCl neutralised by antalid = 0.1387 g
* Amt. of HCl neutralised per gram of antalid = 0.1387 g
Solution: \((\)\(N\) × \(V\)\()_{HCl}\) = \((\)\(N\) × \(V\)\()_{NaOH}\)
(1 x 5 ) = \((\)\(1\) × \(V\)\()_{NaOH}\)
= \(V(NaOH)\) = ( 1 x 5) / 1 = 5ml
In control expt. data \(V(NaOH)\) = 5.6ml
But in given data, \(V(NaOH)\) = 1.2ml
So, Volume diff. of NaOH between back titration and control = 5.6 - 1.2 = 4.4ml
So, given follows
So, 4.4 ml of HCl means,
its Conc. will be equal to, 4.4 x 36.5 / 1000 = 0.1606 g
This is correct in control expt. results
In control expt. data, \(V(NaOH)\) = 5.6ml
This corresponds to 5.6 x 40 / 1000 = 0.224g
This correspond to 0.224 / 40 = 0.0056 moles
This is correct in control expt. results.
Titration is a laboratory technique used to determine the concentration of an unknown solution by reacting it with a known solution. It is a quantitative method used to determine the amount of a substance in a sample. Titration is often used in chemistry to determine the concentration of acids, bases, and salts.
In a titration, a measured amount of the unknown solution is slowly added to a known solution of a substance with a known concentration called the titrant. The titrant is added until the reaction is complete, and a color change or other observable change occurs. The point at which the reaction is complete is known as the endpoint, and it is usually determined using an indicator, which changes color when the reaction is complete.
To learn more about Titration visit here:
brainly.com/question/2728613
#SPJ4
Related Questions
What was Mendeleyev's purpose for
reorganizing the periodic table?
Answers
Answer:Mendeleev realized that the physical and chemical properties of elements were related to their mass in a ‘periodic’ way.
Explanation: He arranged them so that the groups of elements with similar properties fell into vertical columns.
Hope this helped love !!
When a substance an electron it energy which is an reaction. a)Absorbs; creates; chemical b)Gains: gains; oxidation c)Looses; looses; oxidation d)Looses; looses; reduction
Answers
When a substance gains an electron, it undergoes a reduction reaction. In a reduction reaction, a substance gains electrons, which results in a decrease in its oxidation state or an increase in its negative charge.
This process is often associated with the transfer of energy to the substance, as the gained electron brings additional negative charge and potential energy.
Reduction reactions are essential in various chemical and biological processes. For example, in cellular respiration, glucose is oxidized (loses electrons) while oxygen is reduced (gains electrons), leading to the release of energy for cellular activities. Similarly, reduction reactions play a crucial role in many other chemical reactions, such as the reduction of metal ions, the formation of covalent bonds, and the production of certain fuels.
To know more about oxidation state
brainly.com/question/31688257
#SPJ11
Help 65 liters of NaCl is equal to how many grams of NaCl? (Use 2 Significant Figures)
Answers
There are approximately 3.8 x \(10^{6}\) g of Sodium Chloride in 65 liters of the solution.
The number of grams of NaCl in 65 liters of a solution can be determined by using the concentration of the solution. Since the concentration of NaCl is not given, it's not possible to determine the exact number of grams of NaCl in 65 liters of the solution.
However, if we assume that the solution is an aqueous solution of NaCl, we can make an estimate using the density of water. The density of water is approximately 1 g/mL, so 65 liters of water is approximately 65 × 1000 = 65000 mL of water. This means that if the concentration of NaCl in the solution is 1 M, then there would be 65000 mL × 1 M = 65000 moles of NaCl in the solution. Since the molar mass of NaCl is 58.44 g/mole, this would correspond to 65000 moles x 58.44 g/mole = 3.80 × \(10^6\) g of NaCl.
This is a rough estimate, and the actual number of grams of NaCl in 65 liters of solution would depend on the actual concentration of NaCl in the solution. However, if we use 2 significant figures, we can estimate that there are approximately 3.8 × \(10^6\) g of NaCl in 65 liters of the solution.
Learn more about Sodium Chloride:
brainly.com/question/9811771
#SPJ4
Company distributes $136,500 to employees as net pay. The income tax withholdings were $20,200 and the FICA withholdings were $9,025. Total payroll costs to the company for this pay period, excluding any unemployment taxes, was:
Answers
Net pay is the amount paid to employees after deductions like income tax and FICA withholdings.
We need to break down the different components of the payroll costs. Net pay is the amount paid to employees after deductions like income tax and FICA withholdings. In this case, the net pay distributed was $136,500. Income tax withholdings, which are based on the employee's income and tax bracket, were $20,200. FICA withholdings, which include Social Security and Medicare taxes, were $9,025.
To calculate the total payroll costs for this pay period, we need to add the net pay, income tax withholdings, and FICA withholdings. This gives us a total of $165,725. This is the amount that the company paid out to employees for this pay period. However, it's important to note that this total excludes any unemployment taxes.
In summary, the total payroll costs for this pay period, excluding any unemployment taxes, was $165,725. This includes the net pay, income tax withholdings, and FICA withholdings.
To know more about income tax visit: https://brainly.com/question/29622671
#SPJ11
En un experimento hacemos reaccionar a una solución de acido cloridico con el metal cinc. Para ello, hemos usado, 6,5g de cinc y gas hidrogeno. ¿Cual es la masa de gas obtenida? Urgente es para un examen
Answers
Answer:
0.200g de gas son obtenidos
Explanation:
La reacción de ácido clorhídrico, HCl, con Zn es:
2HCl(aq) + Zn(s) → ZnCl₂(s) + H₂(g)
Donde 2 moles de ácido reaccionan con 1 mol de Zn para producir una mol de cloruro de cinc y una mol de hidrógeno (gas)
Asumiendo que el ácido está en exceso, las moles de 6.5g de Zn (Masa molar: 65.38g/mol) son:
6.5g × (1mol / 65.38g) = 0.0994 moles de Zn
Como 1 mol de Zn produce 1 mol de hidrógeno, las moles de hidrógeno son 0.0994 moles de H₂. En gramos (Masa molar H₂ = 2.01g/mol):
0.0994 moles H₂× (2.01g / mol) = 0.200g de gas son obtenidos
Want brainlyest? come and answer this "question"!
What are chemical formulas? Give an example of a chemical formula.
The number of compounds possible are limited by what three factors?
Which individual elements make up the following common compounds?
CaCO3
CaCl2
C3H6O
HCl
Ca(OH)2
Answers
Answer: A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound. here's an example Acetic acid formula CH3COOH. the compound factors it is The electron affinity or the ionising potential. here are elements of making compounds CaCo3 is a Calcium and a carbonate CaCl2 is a calcium and a chlorine
C3H60 is three Calcium’s and 60 hydrogens
HCL is one hydrogen and a chlorine
Ca(OH)2 is one calcium and two hydroxides (OH-).
Explanation: I hope that was helpful !
how do we know that a chemical reaction occured
Answers
Answer: signs of a chemical change are a change in color and the formation of bubbles. The five conditions of chemical change: color change, formation of a precipitate, formation of a gas, odor change, temperature change.
Explanation:
¯\_(ツ)_/¯(⌐■_■)(☞゚ヮ゚)☞☜(゚ヮ゚☜)
The car has a rechargeable battery to drive it’s motor. The rechargeable battery provided a potential difference of 330 volts and can store up to 64 mega Jules it takes 8 hours for the battery to receive a full charge assume that the charging process is 100% efficient calculate the total charge the flows while the battery is being charged
Answers
The total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
To calculate the total charge that flows while the battery is being charged, we can use the relationship between electrical energy, potential difference, and charge.
The electrical energy (E) stored in the battery is given as 64 mega Jules (64 MJ). The potential difference (V) provided by the battery is 330 volts. We know that the energy (E) is equal to the product of the potential difference (V) and the charge (Q):
E = V * Q
Since the charging process is 100% efficient, all the electrical energy supplied is stored in the battery. Therefore, we can rearrange the equation to solve for the charge (Q):
Q = E / V
Substituting the given values, we have:
Q = 64 MJ / 330 V
To perform the calculation, we need to convert mega Jules (MJ) to joules (J) since the SI unit of energy is joules. One mega Joule is equal to 1 million joules:
Q = (64 * 10^6 J) / 330 V
Calculating the division:
Q ≈ 193,939.39 Coulombs
Therefore, the total charge that flows while the battery is being charged is approximately 193,939.39 Coulombs.
This value represents the quantity of electric charge transferred during the charging process, and it indicates the amount of electricity that enters the battery.
For more such questions on charge visit:
https://brainly.com/question/18102056
#SPJ8
what evidence have you discovered to explain how the structure of compounds determens the properties of the compounds
Answers
All of the properties such as ice floating on water, while most solids would sink when placed in its liquid are all due to the structure of the compounds.
The structure of the compounds includes the bonding angle, the type of bonds, the size of the molecule, the interactions between the molecules etc. Slight changes in the chemical structure and affect the properties if the compound.
Isomeric compounds with the same chemical formula but different structures can have different melting and boiling point and differ in reactivity and flammability.
Another common change in isomers are with the double bonds. A double bond can be in the cis formation or in the trans formation, and this will affect its properties as trans isomers will be having high melting point than the cis isomer.
Thus, structure of compounds do determine the properties of the compounds.
To know more about the structure and properties of compounds
https://brainly.com/question/4661963
#SPJ1
Calculate the density of an object with a mass of 20 grams and a volume of
1000 cm3
Answers
Answer:
20/1000=0.02 denity+mass divided by volume
PLEASE HELP ME ILL GIVE BRAINLIEST

Answers
Answer:
TRUE :)
Explanation:
blood is pumped through the pulmonary artery to the lungs, where it picks up oxygen and releases carbon dioxide.
Answer:
False
Explanation:
it takes it to tha lungs.
Which conditions are likely to follow the current conditions in the area you focused on in the previous map? Select three responses. High dew point less humidity no precipitation lower pressure cooler temperatures.
Answers
Answer: 2,3 and 5
Explanation:
Answer:
B. Less humidity
C. No precipitation
E. Cooler temperatures
( Hope this helps ) <3 ( Give person above brainliest )

A fluid is flowing over a heated plate. The viscosity of flowing fluid is 0.001 Pa s and they specific heat at constant pressure is 1 kJ/kgk. The thermal conductivity of the heated plate is 1 W/m⋅k. (a) calculated the prand +1 numbes (b) comment on the relationship b/w hydrodyransic and thermal boundorylayer
Answers
(a) Prandtl number:Prandtl number is a dimensionless quantity, and it represents the ratio of momentum diffusivity to thermal diffusivity. It is usually represented as Pr = v/α, where v is kinematic viscosity and α is thermal diffusivity. It is one of the key parameters that determine the flow characteristics of fluids. The Prandtl number can be determined by the following formula:Pr = μ*Cp/k = 0.001 * 1000 / 1 = 1The Prandtl number is 1.(b) Hydrodynamic and Thermal Boundary Layer:Thermal boundary layer and hydrodynamic boundary layer are the two types of boundary layers. The thermal boundary layer and hydrodynamic boundary layer have different lengths in fluid flow over a flat plate, and the thermal boundary layer is always longer than the hydrodynamic boundary layer because of the low thermal conductivity of the fluid.The ratio of the length of the thermal boundary layer to the length of the hydrodynamic boundary layer can be expressed as:δt/δ = (Pr)^1/2Where δt is the length of the thermal boundary layer, δ is the length of the hydrodynamic boundary layer, and Pr is the Prandtl number. The Prandtl number is greater than 1 for fluids with high viscosity and low thermal conductivity, and the thermal boundary layer is larger than the hydrodynamic boundary layer.Therefore, there is a relationship between the hydrodynamic and thermal boundary layer, and it is directly proportional to the square root of the Prandtl number. In summary, the thermal boundary layer and hydrodynamic boundary layer are related, and the Prandtl number plays a key role in their relationship.
How many oxygen atoms are in Ni(MnO4)2
Answers
Answer:
i think its 8
Explanation:
O4 is 4 oxygen and then its doubled with the 2 at the end
a current of 1.5 a is flowing through a 4 resistor.
Answers
Answer:
than what can i do lol
HELPPPP!!!! SCIENCE!!!!
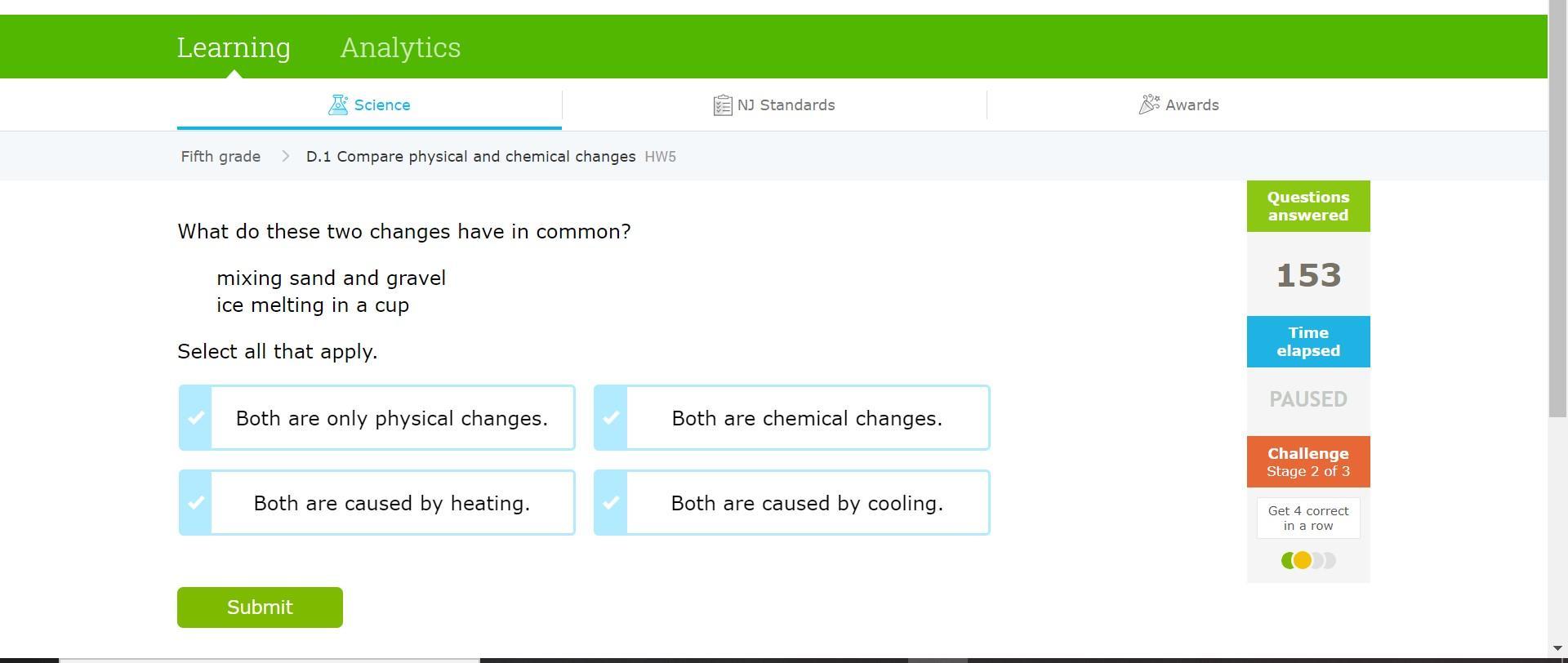
Answers
Answer:
Both are only physical changes
____________ law is a gas law that relates pressure and volume and states that for a fixed amount of gas at constant temperature, the pressure and volume of the gas are ____________ related.
Answers
Boyle's Law relates pressure and volume of a fixed amount of gas at constant temperature; they are inversely related.
Boyle's Law, named after the scientist Robert Boyle, describes the relationship between pressure and volume of a gas. According to this law, if the temperature remains constant, the pressure and volume of a given amount of gas are inversely proportional to each other. This means that as the volume of a gas increases, the pressure decreases, and vice versa.
The mathematical expression for Boyle's Law is P1V1 = P2V2, where P1 and V1 represent the initial pressure and volume, and P2 and V2 represent the final pressure and volume. Boyle's Law is fundamental in understanding the behavior of gases and is used in various applications, such as in the study of gas behavior and in the design of ventilation systems.
Learn more about Boyle's Law here:
https://brainly.com/question/21184611
#SPJ11
If you were to buy a municipal bond for $10,000 with an interest rate of 4% and hold it to its maturity date in 10 years, how often would you receive an interest payment, and for what amount
Answers
When you buy a municipal bond for $10,000 with a 4% interest rate and hold it until maturity in 10 years, you would typically receive interest payments semiannually.
Municipal bonds usually pay interest on a semiannual basis, meaning you receive two payments each year. To calculate the interest payment amount, you would divide the annual interest rate by 2, as you receive payments every six months. In this case, the annual interest rate is 4%, so the semiannual interest rate would be 2% (4% divided by 2). To determine the interest payment amount, you would multiply the bond's face value ($10,000) by the semiannual interest rate (2%). Thus, each interest payment would amount to $200 ($10,000 multiplied by 2%).
Over the course of 10 years, you would receive a total of 20 interest payments (2 payments per year for 10 years). Therefore, the cumulative interest payments would amount to $4,000 ($200 per payment multiplied by 20 payments). It's important to note that municipal bond interest payments and terms can vary, so it's essential to review the specific details of the bond before making any investment decisions.
Learn more about interest rate here:
https://brainly.com/question/14556630
#SPJ11
Can someone tell me what the answer for 7 and 8

Answers
8; The subscript would be 1. Since no subscript indicates 1.
Please brainliest
weight is a flawed measure of mass because it.... PLEASE ANSWER BY 10/30/21 or a. s. a. p.
Answers
Answer:
I believe it's because it's based on gravity
Explanation:
mass is like your true weight while weight is your mass and some equation with gravity. it's been a few years
what happens when you add vinegar to baking soda
Answers
Answer:
When baking soda is mixed with vinegar, something new is formed. The mixture quickly foams up with carbon dioxide gas. If enough vinegar is used, all of the baking soda can be made to react and disappear into the vinegar solution.
Explanation:
Elements on the right side of the Periodic Table are mostly in what state of matter?
Answers
\(\huge\mathfrak\red {Answer:}\)
Elements on the right side of the periodic table are mostly Non - metals.
And Nonmetals exist in all three states of matter. The majority are gases, such as nitrogen and oxygen. Bromine is a liquid. A few are solids, such as carbon and sulfur.
\(\mathfrak\purple {Hope\: this\: helps\: you...}\)
Calculate E0, E, and ΔG for the following cell reactions (a) Mg(s) + Sn2+(aq) ⇌ Mg2+(aq) + Sn(s) where [Mg2+] = 0. 055 M and [Sn2+] = 0. 055 M
Answers
The E° for cell reaction is - 2.37 V and 2.23 V and E for cell reaction = 2.22V and ΔG = - 428.39kJ/mol.
The formula for solving the equation for given cell is as follows :
E°cell , Ecell and Δ\(G_{rnx}\)
The standard cell potential is the potential of cell at standard condition of 1MConcentration and pressure 1 atm E°cell
calculation :
E°cell = E° cathode - E° anode it is calculated using the Nernst equation which is discussed below :
Ecell = E°cell -- \(\frac{RT}{nF}\) 1n K = E°cell -- \(\frac{0.0591}{n}\)log \(\frac{Products}{Reactants}\)
Here, F is the Faraday's constant, R is the gas constant, T is the temperature, and n is the number of transferred electrons. K is the equilibrium constant.
The Gibbs free energy is the greatest work that is finished by a framework . The standard cell potential is without like energy by the recipe as follows;and F is Faraday's steady.
A system's maximum amount of work is referred to as its Gibbs free energy. The standard cell potential is connected with the free energy by the recipe as follows: Δ G = -n F Ecell
Here, E cell is cell potential
Δ G is the free energy n is the quantity of electrons moved and F is Faraday's steady.
The given net cell equation is as follows: Mg + Sn²⁺⇒ Mg²⁺ + SnOxidation :
Mg ⇒ Mg ²⁺ + 2e⁻ E⁰anode = - 2.37 V
Reduction:Sn²⁺ + 2e⁻⇒ Sn E⁰
So, cathode = - 0.14V
The standard cell potential is calculated as follows:E⁰ cell = - 0.14 V- (- 2.37 V ) = 2.23 V
The half reaction potentials for the oxidation and reduction are determined. They are subbed in the equation and the standard cell potential is determined.
Number of electrons transferred , n = 2 ,[Mg²⁺] = 0.055M , [ Sn²⁺ ] = 0.030 M The Nernst equation for reaction :
Ecell = E °cell = \(\frac{0.0591}{n}\)log Mg ²⁺ / Sn²⁺
The cell potential for reaction is :
Ecell = 2.23V - \(\frac{0.0591}{2}\)log\(\frac{0.055M}{0.030M}\)= 2.22V
The values are substituted for the reaction calculated here in the Nernst equation and cell potential.
Calculation for the free energy for reaction ,
] Δ\(G_{rxn}\) = -nFE cell
= - 2 × 96485 C/ mol ×2.22 V
= --428393J/mol × \(\frac{1KJ}{1000J}\) = - 428.39kJ/mol
The cell potential for the response is subbed in the recipe and free energy for the response is determined
Nernst equation :
The standard electrode potential, absolute temperature, the number of electrons involved in the redox reaction, and activities (often approximated by concentrations) of the chemical species undergoing reduction and oxidation, respectively, can all be used to calculate the reduction potential of a half-cell or full cell reaction using the Nernst equation, a chemical thermodynamic relationship.
Learn more about Nernst Equation :
brainly.com/question/15237843
#SPJ4
The combustion of methane is a reaction commonly used in chemistry problems due to its ability to fit into multiple topics. So it should not surprise you to see it here as well. How many L of CO2 would be produced if 45 g of CH4 was combusted with ample oxygen in a room that was 90 degrees Celsius and under 1 atm of pressure
Answers
Answer:
20.76 L OF CO2 WILL BE PRODUCED BY 45 G OF METHANE.
Explanation:
Equation of the reaction:
CH4 + 02 --------> CO2 + 2H20
Molar mass of methane = ( 12+ 1*4) g/mol = 16 g/mol
Calculate the number of moles present in 45 g of methane
1 mole of methane = 16 g / mol of methane
(45 / 16) mole of methane = 45 g of methane
= 2.8125 moles
Using the ideal gas equation:
PV = nRT
P = 1 atm
n = 2.812 moles
T = 90 C
R = 0.082 L atm/ mol C
V = unknown
So we have:
V = nRT / P
V = 2.8125 * 0.082 * 90 / 1
V = 20.756 L
In the production of CO2 by 45 g of methane, 20.756 L of methane was used.
Then, the volume of CO2 produced by this volume will be 20.756 L since 1 mole of methane produces 1 mole of CO2.
In other words;
1 mole of CH4 = 1 mole of CO2
22.4 dm3 of CH4 = 22.4 dm3 of CO2
20.76 DM3 = 20.76 dm3
The volume of CO2 produced will therefore be 20.76 L
How do the physical and chemical properties of the new substance or substances created in the chemical reaction ( the products ) compare to the properties of the elements and original chemicals ( the reactants ) that made it ?
Answers
The table shows data about abiotic factors of streams in two different watersheds.
In general, a stream with a lower temperature and more neutral pH tends to be
healthier. Based on these criteria, which watershed contains the healthier
stream?

Answers
Answer:
B
Explanation:
calculate the [oh-] of a 0.500 m solution of aqueous ammonia. the kb 1.74 x 10-5
Answers
The concentration of [OH-] in a 0.500 M solution of aqueous ammonia is 2.95 x 10^-3 M.
To calculate the [OH-] of a 0.500 M solution of aqueous ammonia, we need to use the equilibrium constant expression for the reaction of ammonia with water:
NH3 (aq) + H2O (l) ⇌ NH4+ (aq) + OH- (aq)
The Kb value given is for the reaction:
NH3 (aq) + H2O (l) ⇌ NH4+ (aq) + OH- (aq)
Kb = [NH4+][OH-]/[NH3]
We are given the concentration of NH3, so we can solve for [NH4+] and [OH-].
Kb = 1.74 x 10^-5
[NH3] = 0.500 M
[NH4+] = [OH-] = x (let the concentration of NH4+ and OH- be x)
Kb = [NH4+][OH-]/[NH3]
1.74 x 10^-5 = x^2/0.500
x^2 = 8.7 x 10^-6
x = 2.95 x 10^-3 M
The [OH-] in a 0.500 M solution of aqueous ammonia is 2.95 x 10^-3 M. The solution is obtained by using the equilibrium constant expression for the reaction of ammonia with water. The Kb value given is used to solve for the concentration of NH4+ and OH-. The [OH-] concentration is equal to the concentration of NH4+. By using this method, we can easily calculate the [OH-] of aqueous ammonia solutions with different concentrations.
To know more about ammonia visit:
https://brainly.com/question/29519032
#SPJ11
What happens to atomic radius on going from left to right in a period in a periodic table?
A. Remains constant
B. Decreases first and then remains constant
C. Decreases
D. Increases
Answers
Please give brainliest I need two more!!!!!!!!!!!!!!!
Predict the nature of the indicated covalent bond
H-Br
I’ll put a picture so it makes more since.
Please Help

Answers
Answer:
It is polar bond
The description is given below u will be more clear about it
Explanation:
Non polar bonds form between two atoms that share their electrons equally. Polar bonds form when two bonded atoms share electrons unequally.
Hope it will help :)
for a 30% wt al - 70% wt si alloy at 600 c what phases are present? what are compositions of all phases present?
Answers
The compositions of all phases present at 600°C, a 30% wt Al - 70% wt Si alloy consists of two phases: α-Al phase with approximately 10% wt Si and Si phase with approximately 2% wt Al.
To determine the phases present and their compositions, we need to consult the Al-Si phase diagram.
1. Locate the Al-Si phase diagram and find the 600°C isotherm line.
2. Identify the 30% wt Al - 70% wt Si composition point on the diagram and see where it intersects the 600°C isotherm line.
3. Determine the phases present at this intersection point.
4. Read the compositions of each phase from the phase boundaries around the intersection point.
From the Al-Si phase diagram at 600°C, the 30% wt Al - 70% wt Si alloy falls within the α-Al solid solution phase, which is primarily aluminum with dissolved silicon, and the Si phase, which is primarily silicon with dissolved aluminum.
For the α-Al phase composition, read the weight percentage of Si in α-Al from the phase boundary on the left side of the intersection point. This value is approximately 10% wt Si in α-Al.
For the Si phase composition, read the weight percentage of Al in Si from the phase boundary on the right side of the intersection point. This value is approximately 2% wt Al in Si.
In summary, at 600°C, a 30% wt Al - 70% wt Si alloy consists of two phases: α-Al phase with approximately 10% wt Si and Si phase with approximately 2% wt Al.
More on alloys: https://brainly.com/question/29799463
#SPJ11