Answers
Related Questions
When heating a solution to boiling on a hot plate, start by ___________. Then, turn the heat to ___________to start. If necessary, __________after waiting about ten minutes without seeing boiling.
Answers
Answer:
1. starting and stabilizing the stir function
2. a medium heat
3.turn up the heat setting
Explanation:
A chemical reaction can be defined as a chemical process which typically involves the transformation or rearrangement of the atomic, ionic or molecular structure of an element through the breakdown and formation of chemical bonds to produce a new compound or substance.
Some of the laboratory apparatus (equipment) used for conducting a chemical reaction are conical flask, Bunsen burner, beaker, tongs, crucible, round bottom flask etc.
When heating a solution to boiling on a hot plate, start by starting and stabilizing the stir function. Then, turn the heat to a medium heat to start. If necessary, turn up the heat setting after waiting about ten minutes without seeing boiling.
The safety precautions that must be taken when heating a solution to boiling on a hot plate;
I. A proper inspection of the round bottom flask for cracks, irregularities or any imperfection.
II. Ensure you avoid heating the flask while it is closed.
III. When suspending the flask on a hot plate, you should ensure that you use a clamp for stability.
Consider the following reaction:
2N2(g) + O2(g) ⇌ 2NO2(g)
A reaction mixture initially contains 3.21 atm N2 and 6.21 atm O2. Determine the equilibrium pressure of NO2 if Kp for the reaction at this temperature is 3.2 × 10-28.
Answers
The equilibrium pressure of the nitrogen oxide is given as 4 atm.
What is the Kp?Kp is the equilibrium constant for a chemical reaction in terms of partial pressures. It is defined as the ratio of the product of the partial pressures of the products raised to their stoichiometric coefficients to the product of the partial pressures of the reactants raised to their stoichiometric coefficients
We know that;
Kp = \(pNO_{2} ^2/pN_{2} ^2 . pO_{2}\)
\(3.2 * 10^-28 = (2x)^2/(3.21 - 2x) (6.21 - x)\\3.2 * 10^-28 = 4x^2/19.9 - 3.21x - 12.42x + 2x^2\\3.2 * 10^-28(19.9 - 15.63x + 2x^2) = 4x^2\\6.4 * 10^-27 - 5 * 10^-27 x + 6.4 * 10^-28x^2 = 4x^2\\x = 4 atm\)
Learn more about Kp:https://brainly.com/question/30550192
#SPJ1
Translate this word equation into a formula equation:
Barium + Gold (II) Phosphate -> Gold +
Barium Phosphate
Answers
Some properties of substance X are listed. ●It conducts electricity when molten. ●It has a high melting point. ●It burns in oxygen and the product dissolves in water to give a solution with pH 11. what is X? A a covalent compound B a macromolecule C a metal D an ionic compound
Answers
An ionic compound is one that dissolves in water to produce a solution with a pH of 11, has a high melting point, conducts electricity when molten, and burns in oxygen.
What are ionic compounds, exactly?Positively charged ions, which are sometimes called as cations, and negatively charged ions, also called as anions together constitute an ionic compound, which are neutral substances. The name of the cation is written first, followed by the name of the anion, in case of binary ionic compounds (ionic compounds that only contain two types of elements).
What is the difference between covalent and ionic compounds?An atom can establish bonds with other atoms in two main ways: covalent and ionic. Sharing of electrons between two or more atoms is involved in case of covalent bonding. When two or more ions come together, they can form ionic bonds that are held together by charge differences.
To learn more about ionic compound visit:
brainly.com/question/29005103
#SPJ1
Suppose the O2 gas evolved by a certain chemical reaction taking place at 35°c is collected over water, using an apparatus something like that in the sketch, and the final volume of gas is collection tube is measured to be 124mL. Calculate the mass of O2 that is in the collection tube round your answer to two significant digits. You can make any normal reasonable assumption about the reaction conditions, and the nature of the gases.

Answers
From the calculations, the mass of the oxygen molecules that we can collect in this case is 0.16 g.
What is the ideal gas equation?The ideal gas equation, also known as the equation of state of an ideal gas, is a mathematical relationship that describes the behavior of an ideal gas. The equation is given by:
PV = nRT
If we assume that the pressure is the same as the atmospheric pressure then;
PV = nRT
n = PV/RT
n = 1 * 0.124/0.082 * 308
n = 0.0049 moles
Mass of the O2 = 0.0049 moles * 32 g/mol
= 0.16 g
Learn more about moles:https://brainly.com/question/26416088
#SPJ1
The graph shows the altitude and temperature for different layers of Earth’s atmosphere. Based on the diagram, which of the following identifies a characteristic of the atmosphere between the stratopause and the tropopause?
answer choices
Cloud formation occurs in the stratosphere.
The warmest air is found in the mesosphere.
The ozone layer is in the stratosphere.
The troposphere is the closest layer to the sun.

Answers
The ozone layer is present in the stratosphere layer of the Earth's atmosphere. Therefore, option (C) is correct.
What is the atmosphere?An atmosphere can be defined as layers of gases that envelop a planet and is held in place due to the gravity of the planetary body. A planet contains an atmosphere when the gravity is great and the temperature of the atmosphere of any planet is low.
Earth's atmosphere is made of Nitrogen (78 %), Oxygen (21%), Argon (0.9%), and Carbon dioxide (0.04 %). The troposphere can be defined as the lowest layer of the atmosphere. The troposphere contains 75 to 80 % of the mass of the atmosphere.
The stratosphere contains the ozone layer, at an altitude between 15km and 35km. This atmospheric layer absorbs most of the UV radiation that Earth receives from the Sun.
Learn more about the atmosphere, here:
https://brainly.com/question/29379794
#SPJ1
What type of alloy will C and Fe form?
A. Substitutional alloy
B. Structural alloy
C. Composite
D. Interstitial alloy
Answers
Write an ionic equation to show the dissociation of ions in aqueous copper(II) bromide.
Answers
Answer:
The ionic equation to show the dissociation of ions in aqueous copper(II) bromide is:
CuBr2 (aq) → Cu2+ (aq) + 2Br- (aq)
In this equation, CuBr2 represents the copper(II) bromide compound that dissociates into Cu2+ and Br- ions when it is dissolved in water (represented by "aq" for aqueous). The Cu2+ ion has a positive charge of 2+ and the Br- ion has a negative charge of 1-.
The total number of atoms in 3.75 mol of ammonium nitrate, NH4NO3 , is
Answers
The total number of atoms in 3.75 mol of ammonium nitrate, NH4NO3, is equal to the Avogadro's number, which is 6.022 x 10^23, times the number of moles, which is 3.75 in this case.
Thus, the total number of atoms in 3.75 mol of ammonium nitrate is equal to 2.26 x 10^24 atoms.
This number can be further broken down into the number of atoms of each element present in the compound. Ammonium nitrate is composed of two elements, nitrogen (N) and hydrogen (H). For each mole of ammonium nitrate, there are 4 moles of hydrogen atoms and 2 moles of nitrogen atoms.
Therefore, in 3.75 moles of ammonium nitrate, there are 15 moles of hydrogen atoms and 7.5 moles of nitrogen atoms, which is equal to 9 x 10^24 hydrogen atoms and 4.05 x 10^24 nitrogen atoms.
Learn more about ammonium nitrate at:
https://brainly.com/question/5148461
#SPJ1
Calcium chloride (a solid ionic salt formed from a group 2A metal and group 7A Halogen) is dissolved into a water solvent. Can the calcium chloride be separated from the solution, and will it retain its properties?
a
No, it cannot be separated through any means. It does not retain its original properties.
b
Yes, it can be separated through evaporation. It does not retain its original properties.
c
No, it cannot be separated through any means. It retains its original properties.
d
Yes, it can be separated through evaporation. It retains its original properties.
Answers
Answer:
Yes, it can be separated through evaporation. It retains its original properties.
Explanation:
Balance the following equation:
C2H5NH2(g) + O2(g) →
CO2(g) + H2O(g) + N2(g)
Answers
Answer:
4C2H5NH2(g) + 15O2(g) → 8CO2(g) + 14H2O(g) + 2N2(g)
Explanation:
The balanced chemical equation for the given chemical reaction is 4 C₂H₅NH₂(g) + 15 O₂(g) → 8 CO₂(g) + 14 H₂O(g) + 2 N₂(g).
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equation,here:
https://brainly.com/question/28294176
#SPJ2
can someone help me with my chemistry homework please???

Answers
1.) Lithium and Sulfide:
Formula: \(\bold{Li_{2}S}\)Ion Charges: \(\bold{Li~1+,~Li~1+,~S~2-}\)2.) Lithium and Chlorine:
Formula: \(\bold{2LiCl}\)Ion Charges: \(\bold{Li~1+, Li~1+,Cl~1-,Cl~1-}\)3.) Lithium and Oxygen:
Formula: \(\bold{Li_{2}O}\)Ion Charges: \(\bold{Li~1+,Li~1+,O~2-}\)4.) Lithium and Nitrogen:
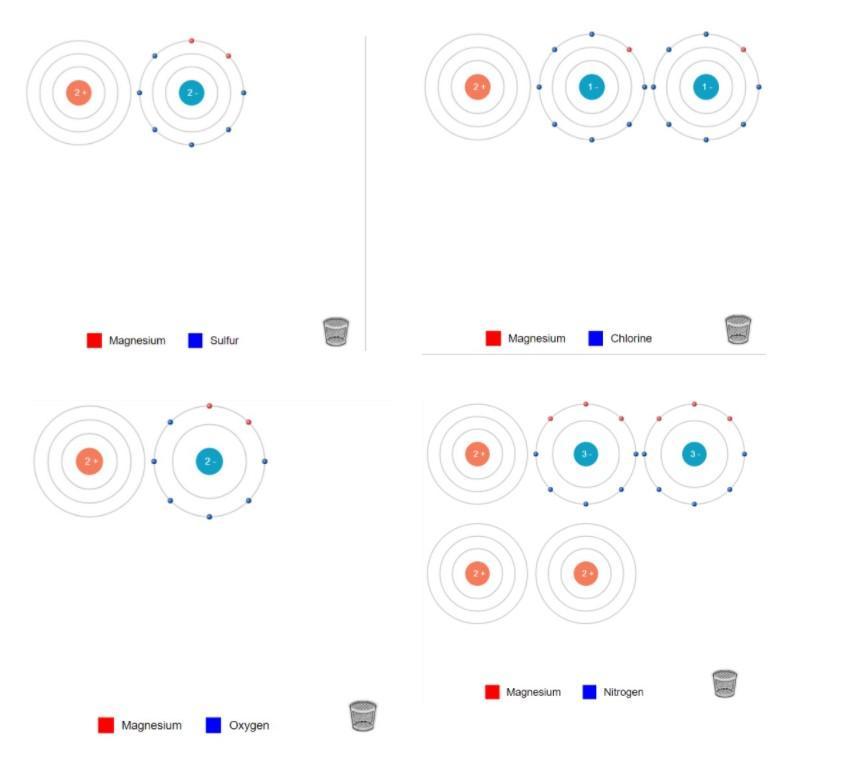
Formula: \(\bold{Li_{3}N}\)Ion Charges: \(\bold{Li~1+,Li~1+,Li~1+,N~3-}\)5.) Magnesium and Sulfur:
Formula: \(\bold{MgS}\)Ion Charges: \(\bold{Mg~2+,S~2-}\)6.) Magnesium and Chlorine:
Formula: \(\bold{MgCl_2}\)Ion Charges: \(\bold{Mg~2+,Cl~1-,Cl~1-}\)7.) Magnesium and Oxygen:
Formula: \(\bold{MgO}\)Ion Charges: \(\bold{Mg~2+,O~2-}\)8.) Magnesium and Nitrogen:
Formula: \(\bold{Mg_3N_2}\)Ion Charges: \(\bold{Mg~2+,Mg~2+,Mg~2+,N~3-,N~3-}\)Explanation:______________________________
Lithium and Sulfur: In order to make Lithium Sulfide, There must be 2 Lithium and 1 Sulfur. You transfer the electrons from both Lithium's to the Sulfur.Lithium and Chlorine:In order to make Lithium Chloride, There must be 2 Lithium and 2 Chlorine. You transfer the electrons from both Lithium's to the Chlorines, (One electron for each chlorine.)Lithium and Oxygen:In order to make Lithium Oxide, There must be 2 Lithium and 1 Oxygen. You transfer the electrons from both Lithium to Oxygen. Lithium and Nitrogen:In order to make Lithium Nitride, There must be 3 Lithium and 1 Nitrogen. You transfer the electrons from all 3 Lithium to Nitrogen. Magnesium and Sulfur:In order to make Magnesium Sulfide, There must be 1 Magnesium and 1 Sulfur. You transfer the both electrons from Magnesium to Sulfur. Magnesium and Chlorine:In order to make Magnesium Chloride, There must be 1 Magnesium and 2 Chlorine. You transfer on electron to each Chlorine. Magnesium and Oxygen:In order to make Magnesium Oxide, There must be 1 Magnesium and 1 Oxygen. You transfer both electrons from Magnesium to Oxygen. Magnesium and Nitrogen:In order to make Magnesium Nitride, There must be 3 Magnesium and 2 Nitrogen. You transfer 3 electrons from Magnesium to Nitrogen.______________________________

What is chemistry?
A. The scientific study of matter
B. The study of living things
C. The study of how matter and energy interact
D. The study of changing ideas
Answers
Answer:
the scientific study of matter i believe
Explanation:
because the definition says the branch of science that deals with the identification of the substances of which matter is composed
6.Observe the equation below 2 Fe2O3(s) + CO(g) Fe(s) + CO2(g)
i) Balance the equation
Answers
Answer:
the given equation is
Fe2o3+co-- Fe+co2
Balanced chemical equation is
Fe2o3 + 3co-- 2Fe+3co2
. How many moles of oxygen (O) are in 1 mole of calcium carbonate (CaCO3)?
Answers
In 1 mole of calcium carbonate, there are approximately 18.066 x 10^23 moles of oxygen.
To determine the number of moles of oxygen (O) in 1 mole of calcium carbonate (CaCO3), we need to examine the chemical formula of calcium carbonate and identify the number of oxygen atoms present.
The chemical formula of calcium carbonate is CaCO3. In this formula, we have one calcium atom (Ca), one carbon atom (C), and three oxygen atoms (O).
The subscript numbers in the formula indicate the number of atoms for each element. Therefore, we have:
1 calcium atom (Ca)
1 carbon atom (C)
3 oxygen atoms (O)
To calculate the number of moles of oxygen in 1 mole of calcium carbonate, we multiply the number of oxygen atoms (3) by the Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of moles of oxygen (O) in 1 mole of calcium carbonate (CaCO3) = 3 moles of oxygen x (6.022 x 10^23 molecules/mol)
Calculating this value, we find:
Number of moles of oxygen (O) in 1 mole of calcium carbonate (CaCO3) ≈ 18.066 x 10^23 moles of oxygen
for more such questions on calcium
https://brainly.com/question/12216405
#SPJ8
(GIVING BRAINLIEST)Match each of the substances below to the the intermolecular force that are present in the substance.
A.) dispersion forces 1.)CBr2Cl2
B.) dipole dipole Force. 2.)NaCl & H2O
C.) hydrogen bonding. 3.)NH3
D.) Ion Dipole Force. 4.)CH4
Answers
Answer:
A4
B1
C3
D2
Explanation:
A4: dispersion forces occur in everything as it is variations in the electron cloud. They are very weak and are the only intermolecular force in a non-polar molecule such as CH4.
B1: Dipole Dipole forces occur between polar molecules. The polarity of the molecule creates a dipole which can attract each other. they are stronger then dispersion forces. CBr2Cl2 is a polar molecule.
C3: hydrogen bonding is a special type of dipole-dipole force and are pretty strong. They can only occur between H-N, H-O, and H-F bonds. NH3 has N-H bonds.
D2: ion dipole forces occur between a polar molecule and ions. They mainly occur when charged species (ions) are in a polar solvent but there are likely less common examples.
I hope this helps. Let me know if anything is unclear.
2Fe(s) +3H2SO4(aq) →Fe2(SO4)3(aq) +3H2(g)When 10.3 g of iron are reacted with 14.8 moles of sulfuric acid, what is the percent yield if 5.40 g of "hydrogen gas" are collected?
Answers
Answer:
1040%
Explanation:
To solve this question we must convert the mass of Iron to moles in order to find limiting reactant. With limiting reactant we can find the theoretical moles of hydrogen and theoretical mass:
Percent yield = Actual yield (5.40g) / Theoretical yield * 100
Moles Fe -Molar mass: 55.845g/mol-:
10.3g * (1mol / 55.845g) = 0.184 moles of Fe will react.
For a complete reaction of these moles there are necessaries:
0.184 moles Fe* ( 3 mol H2SO4 / 2 mol Fe) = 0.277 moles H2SO4.
As there are 14.8 moles of the acid, Fe is limiting reasctant.
The moles of H2 produced are:
0.184 moles Fe* ( 3 mol H2 / 2 mol Fe) = 0.277 moles H2
The mass is:
0.277 moles H2 * (2.016g/mol) = 0.558g H2
Percent yield is:
5.40g / 0.558g * 100 = 1040%
It is possible the experiment wasn't performed correctly
pls help!!
The number that represents a neutral pH is ________.
Answers
Answer:
7
Explanation:
A sample of iron absorbs 67.5 J of heat, upon which the temperature of the sample
increases from 21.5 °C to 28.5 °C. If the specific heat of iron is 0.450 J/g OC, what is
the mass (in grams) of the sample?
Answers
Explanation:
Observe the units of all of the prameters to solve this problem
m * (28.5-21.5)C * .450 J/(gC) = 67.5 J
m * 7 * .450 = 67.5
m = 67.5/(7 * .450) = 21.4 grams
A scientific law is different from a scientific theory because it describes something in nature without attempting to explain it.
Answers
Yes, that statement is generally correct. A scientific law is a statement that describes a phenomenon or pattern in nature, often expressed mathematically, without attempting to explain why it occurs. A scientific theory, on the other hand, is a well-substantiated explanation for a set of phenomena, based on empirical evidence and scientific reasoning.
A scientific law summarizes what happens in a particular situation, often in the form of an equation or formula, whereas a scientific theory attempts to explain why it happens.
For example, the law of gravity describes the attraction between masses, but it does not explain why this attraction occurs. In contrast, the theory of general relativity attempts to explain the underlying principles of gravity, including its effects on the curvature of space-time.
It's worth noting that both scientific laws and scientific theories are based on empirical evidence, but they serve different purposes in scientific inquiry. Laws describe what happens in a particular situation, while theories attempt to explain why it happens.
For more question on scientific law click on
https://brainly.com/question/16347879
#SPJ11
oxidation number of Ag in Ag2O
Answers
The oxidation number of Ag in Ag2O is +1.
In Ag2O, there are two silver atoms (Ag) and one oxygen atom (O). Oxygen is known to have an oxidation number of -2 in most compounds. Since the compound is neutral, the sum of the oxidation numbers of all the atoms must equal zero.
Therefore, the oxidation numbers of the two silver atoms must add up to +2 to balance out the -2 oxidation number of the oxygen atom. Since there are two silver atoms, each silver atom must have an oxidation number of +1 to yield a total oxidation number of +2 for the compound.
In Ag2O, the silver atoms lose one electron each to form Ag+ ions. This results in an oxidation number of +1 for each silver atom. The oxygen atom gains two electrons from the silver atoms to achieve a stable octet configuration, resulting in an oxidation number of -2 for the oxygen atom. The compound Ag2O is formed through the transfer of electrons, with each silver atom exhibiting an oxidation number of +1.
for such more questions on oxidation
https://brainly.com/question/13182308
#SPJ8
The gas tank of car hold 22.3 gallons.If the density of gasoline is 0.8206g/mL,determine the massin kilograms and pounds of the fuelin a full truck
Answers
Density = mass/Volume or D = m/V
Given: V = 22.3 gal
D = 0.8206 g/mL
1) Rearrange the equation to solve for m
2) Find conversion factor(s) and convert V from gal to mL (by dimensional analysis) so the units cancel
3) Solve for m in g
4) Find conversion factor(s) and convert m from g → lbs
1) D = m/V rearranges to m = D ∗ V
2) 1 gal = 3.785 L and 1 L = 1000 mL; 22.3 gal ∗ 3.785 L/1 gal ∗ 1000 mL/1 L = 84 406 mL
3) m = 0.8206 g/mL ∗ 84 406 mL = 69 260 g
4) 1 lb = 453.6 g; 69 260 g ∗ 1 lb/453.6 g = 153 lbs
Learn More about Density:
https://brainly.com/question/24386693
Which statement describes a digital signal?
Answers
Answer:
digital signal
Answer:
Its made of numbers
Explanation:
did the assignment, hope this helps :]
list 5 island in the Philippines
Answers
Answer:
Coron, Palawan, El Nido, Palawan, Cebu, Boracay, Siargao.
Explanation:
I hope this helps.
coron,palawan,El nidu, Cebu and boracay are 5 island in Philippines.....
How much heat must be added to a 34.2 g sample of aluminum in order to raise the temperature of the aluminum 34 oC? (The specific heat of Aluminum is 0.9 J/g oC)
Answers
The amount of heat required to raise the temperature of the 34.2 g sample of aluminum by 34 oC is 1043.52 J.
What is Temperature?
Temperature is a measure of the average kinetic energy of the particles in a substance. It is a physical property that determines the direction of heat flow between two objects or systems in contact with each other. Temperature is measured in degrees Celsius (°C) or Fahrenheit (°F), or in kelvin (K) in the International System of Units (SI).
The amount of heat (q) required to raise the temperature of a substance can be calculated using the formula:
q = m x c x ΔT
Where:
m = mass of the substance (in grams)
c = specific heat of the substance (in J/g oC)
ΔT = change in temperature (in oC)
Plugging in the values given:
m = 34.2 g
c = 0.9 J/g oC
ΔT = 34 oC
q = (34.2 g) x (0.9 J/g oC) x (34 oC)
q = 1043.52 J
Therefore, the amount of heat required to raise the temperature of the 34.2 g sample of aluminum by 34 oC is 1043.52 J.
Learn more about Temperature
brainly.com/question/26866637
#SPJ1
Write the electron configuration for each of the following ions: (a) As3– (b) I– (c) Be2+ (d) Cd2+ (e) O2– (f) Ga3+ (g) Li+ (h) N3– (i) Sn2+ (j) Co2+ (k) Fe2+ (l) As3+
Answers
Answer:
a) 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶
b) 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶
c) 1s²
d) 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 4d¹⁰
e) 1s² 2s² 2p⁶
f) 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰
g) 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰
h) 1s² 2s² 2p⁶
j) 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 4d¹⁰
k) 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁶
l) 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰
An unknown liquid has a heat of vaporization of 5.48 kJ/mole. If the vapor pressure of this liquid at -170 degrees C is 117 torr, what is the normal boiling point of this liquid in degrees C? HINT: Normal boiling point occurs when the vapor pressure of the liquid is the same as atmospheric pressure (1 atm or 760 mm Hg).
Answers
The normal boiling point of the unknown liquid is 57.4°C.
The normal boiling point occurs when the vapor pressure of the liquid is equal to the atmospheric pressure. At normal boiling point, the temperature of the liquid is called the boiling point.
Using the Clausius-Clapeyron equation:
ln(P₂/P₁) = -(ΔHvap/R) * (1/T₂ - 1/T₁)
where P₁ is the vapor pressure at the given temperature T₁, P₂ is the vapor pressure at the boiling point temperature T₂, ΔHvap is the heat of vaporization, R is the gas constant.
At -170°C, the vapor pressure of the liquid is given as P₁ = 117 torr. At normal boiling point, the vapor pressure of the liquid is P₂ = 760 torr.
Converting all units to SI units, we have:
P₁ = 15.47 Pa
P₂ = 101325 Pa
ΔHvap = 5480 J/mol
R = 8.314 J/(mol*K)
Plugging in the values, we get:
㏑(101325/15.47) = -(5480/8.314) * (1/T₂ - 1/103.15)
Solving for T₂, the boiling point is found to be:
T₂ = 57.4°C
As a result, the unknown liquid's usual boiling point is 57.4°C.
To know more about the Pressure, here
https://brainly.com/question/14748171
#SPJ1
How can you use titration to determine an unknown concentration?
Answers
Answer:
You will use the volume and known concentration of the titrant to calculate the number of moles needed to titrate a sample of unknown concentration
Please i meed help quick and thank you

Answers
It is the 4th scenario is the dependent event. There are 7 gold tokens and 4 silver tokens in a cup. The first student randomly draws a gold token and keeps it. A second student randomly draws a gold token from the cup.
How did we identify the dependent event?The fouth scenario is a dependent event because the probability of the second student drawing a gold token is affected by the outcome of the first student's draw.
If the first student draws a gold token, then there are only 6 gold tokens left in the cup, the probability changes. but if the first student does not draw a gold token, then there are 7 gold tokens left in the cup, the probability will remain the same
Find more exercises on dependent events;
https://brainly.com/question/11473170
#SPJ1
500.0 liters of a gas in a flexible-walled container are prepared at 0.92 atm and 473K. The gas is placed into a tank under high pressure. When the tank cools to 293K, the pressure of the gas is 3.0 atm. What is the volume of the gas?
P1V 1 T2=P 2 V 2 T 1
Question 17 options:
48 L
248 L
19 L
95 L
Answers
The volume of the gas in the tank at 293K and 3.0 atm pressure is 248 L. Hence, option B is correct.
Given:
T1 = 473K
T2 = 293K
P1 = 0.92 atm
P2 = 3.0 atm
The ideal gas law equation is:
PV = nRT
where P is pressure, V is volume, n is the number of moles of gas, R is the universal gas constant, and T is temperature.
n1 = (PV)/(RT)
= (0.92 atm × 500.0 L)/(0.0821 Latm/molK × 473K)
= 10.42 mol
Use the ideal gas law again to find the final volume of the gas in the tank under high pressure:
V2 = (n1 × R × T2)/P2
= (10.42 mol × 0.0821 Latm/molK × 293K)/(3.0 atm)
= 248 L
Therefore, the volume of the gas in the tank at 293K and 3.0 atm pressure is 248 L.
To learn more about pressure, follow the link:
https://brainly.com/question/31525061
#SPJ1
Answer:
Using the ideal gas law, PV=nRT, where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin, we can solve for n:
n = PV/RT
We know that the initial volume is 500.0 L, pressure is 0.92 atm, and temperature is 473K. We can use this information to find the initial number of moles:
n1 = (0.92 atm x 500.0 L)/(0.08206 L atm/mol K x 473K) = 11.80 mol
Next, we can use the ideal gas law again to find the final volume. We know that the final pressure is 3.0 atm and the final temperature is 293K:
V2 = nRT2/P2
V2 = (11.80 mol x 0.08206 L atm/mol K x 293K)/3.0 atm = 95 L
Therefore, the volume of the gas at the lower temperature and higher pressure is approximately 95 L. Answer: 95 L.