for a particular liquid, raising its temperature from 25 °c to 45 °c causes its vapor pressure to double. what is the enthalpy of vaporization of this liquid?
Answers
The enthalpy of vaporization of this liquid is 34.2 kJ/mol.
The enthalpy of vaporization of a liquid can be calculated using the Clausius-Clapeyron equation, which is:
ln(P₂/P₁) = (-ΔHvap/R)(1/T₂ - 1/T₁)
Where:
P₁ and P₂ are the vapor pressures at temperatures T₁ and T₂, respectively
ΔHvap is the enthalpy of vaporization
R is the gas constant (8.314 J/mol·K)
Given that the vapor pressure of the liquid doubles when its temperature is raised from 25 °C to 45 °C, we can plug in the values into the equation and solve for ΔHvap:
ln(2P₁/P₁) = (-ΔHvap/8.314)(1/318 - 1/298)
ln(2) = (-ΔHvap/8.314)(0.002013)
ΔHvap = -(8.314)(ln(2)/0.002013)
ΔHvap = 34.2 kJ/mol
To know more about enthalpy of vaporization click on below link:
https://brainly.com/question/30258840#
#SPJ11
Related Questions
Name the following hydrocarbon compounds.



Answers
The naming of compound can be obtained by following the IUPAC principle. This is shown below:
For the 1st diagram:
Locate the longest continuous carbon chain. In this case it is carbon 5. Hence, the parent name is pentaneIdentify the substituent groups attached. In this case the substituent groups attached is two methyl, CH₃ Give the substituents the best possible low count. In this case, the two CH₃ is located at carbon 3Combine the above to obtain the IUPAC name for the compound.Thus, the IUPAC name for the compound is: 3,3-dimethylpentane
For the 2nd diagram:
Locate the longest continuous carbon chain. In this case it is carbon 5. Hence, the parent name is pentene since it contains a double bondIdentify the substituent groups attached. In this case there are no substituent groups attached.Thus, the IUPAC name for the compound is: pentene
For the 3rd diagram:
Locate the longest continuous carbon chain. In this case it is carbon 6. Hence, the parent name is hexaneIdentify the substituent groups attached. In this case the substituent groups attached is three methyl, CH₃ Give the substituents the best possible low count. In this case, two CH₃ are located at carbon 2 while the 3rd is located at carbon 3Combine the above to obtain the IUPAC name for the compound.Thus, the IUPAC name for the compound is: 2,2,3-trimethylhexane
Learn more about IUPAC name:
https://brainly.com/question/23881815
#SPJ1
50 ml of 0.600 m sr(no3)2 with 50 ml of 1.60 m kio3 caculatte the equilibreum sr2
Answers
The equilibrium Sr2+ is 0.15 M.
The chemical reaction that occurs when 50 ml of 0.600 M Sr(NO3)2 reacts with 50 ml of 1.60 M KIO3 is: 2 Sr(NO3)2 + 2 KIO3 → Sr(IO3)2 + 2 KNO3From this balanced equation, it can be seen that 2 moles of Sr(NO3)2 produce 1 mole of Sr(IO3)2.
Therefore, moles of Sr(NO3)2 present initially = 0.600 × 0.050 = 0.03 mol Moles of KIO3 present initially = 1.60 × 0.050 = 0.08 mol
Since the ratio of moles of Sr(IO3)2 to Sr(NO3)2 is 1:2, therefore moles of Sr(IO3)2 formed = 0.03 / 2 = 0.015 mol
The final volume of the mixture is 50 + 50 = 100 ml
Number of moles of Sr(IO3)2 in 100 ml solution = 0.015 mol
Molarity of Sr(IO3)2 = (Number of moles of Sr(IO3)2) / (Volume of solution in L) = (0.015 mol) / (0.100 L) = 0.15 M
Therefore, the equilibrium Sr2+ is 0.15 M.
Know more about equilibrium :
https://brainly.com/question/30325987
#SPJ11
The equilibrium Sr²⁺ concentration in the solution will be approximately 0.600 mol/L.
To calculate the equilibrium Sr²⁺ concentration in the solution, we need to determine whether a precipitation reaction occurs between Sr(NO₃)₂ and KIO₃, and if so, how much Sr²⁺ precipitates.
The balanced chemical equation for the precipitation reaction between Sr(NO₃)₂ and KIO₃ is;
2Sr(NO₃)₂ + KIO₃ → Sr(IO₃)₂ + 2KNO₃
We can see that for every 2 moles of Sr(NO₃)₂, 1 mole of Sr(IO₃)₂ precipitates.
First, let's calculate the moles of Sr(NO₃)₂ and KIO3 in the solution;
Moles of Sr(NO₃)₂ = Volume (L) × Concentration (M)
= 0.050 L × 0.600 M
= 0.030 mol
Moles of KIO₃ = Volume (L) × Concentration (M)
= 0.050 L × 1.60 M
= 0.080 mol
From the balanced equation, we can see that the limiting reagent is Sr(NO₃)₂ because it has fewer moles than KIO₃.
Since 2 moles of Sr(IO₃)₂ precipitate for every 2 moles of Sr(NO₃)₂, we can conclude that all the Sr(NO₃)₂ will react and form Sr(IO₃)₂.
Now, let's calculate the concentration of Sr²⁺ ions in the solution after the reaction:
The total volume of the solution is 50.0 mL + 50.0 mL = 0.100 L
Since 2 moles of Sr(NO₃)₂ give 2 moles of Sr²⁺ ions, and we have 0.030 mol ofSr(NO₃)₂;
Concentration of Sr²⁺ ions = Moles of Sr²⁺ ions/Volume of the solution
= (2 × 0.030 mol) / 0.100 L
= 0.600 M
Therefore, the equilibrium Sr²⁺ concentration in the solution is 0.600 mol/L.
To know more about precipitation reaction here
https://brainly.com/question/29762381
#SPJ4
--The given question is incomplete, the complete question is
"A solution is prepared by mixing 50.0 mL of 0.600 M Sr(NO₃)₂ with 50.0 mL of 1.60 M KIO₃. Calculate the equilibrium Sr²⁺ concentration in mol/L for this solution. Ksp for Sr(IO₃)₂ = 2.30E-13."--
If a substance has a mass of 0.00235 grams and you need the mass in kilograms, will the number appear to become smaller or larger?
Answers
Answer: shorter
Explanation:
In a _____ molecules move just enough to stay close together but not to stick
Answers
Answer:
I think it might be liquid
Answer: in a molecular molecules move just enough to stay close together but not to stick
Explanation:
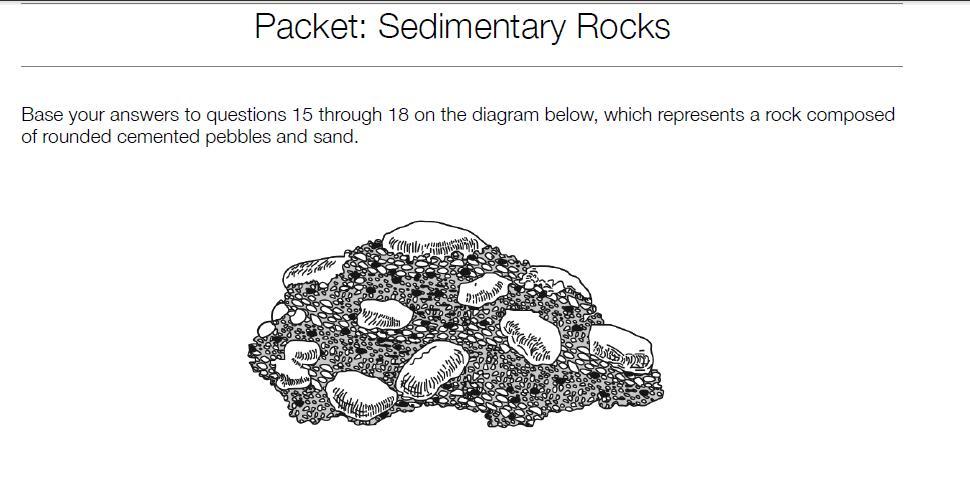
What is the name of the rock?
a. limestone
b. breccia
c. sandstone
d. conglomerate
Answers
Answer:B
Explanation:
A cylinder of volume 2.00 L contains 0.100 mol He(g) at 30°C. Which process does more work on the system, compressing the gas isothermally to 1.00 L with a
constant external pressure of 5.00 atm or compressing it reversibly and isothermally to the same final volume?
Answers
The system's effort in the process known as isothermal can be determined using the equation. W = 2.303RT log 10 (V 2 / V 1), where V is the volume in two distinct states that are both at temperature T, and R is the constant of all gases.
What is the isothermal mechanism of a refrigerator?Although a refrigerator's mechanism goes through a number of changes, the internal temperature never varies. Here, warmth energy is released and spread throughout the nearby area. The heat pump is an additional instance of an isothermal mechanism.
What does adiabatic compression mean?Isothermal compression is a thermodynamic mechanism that lowers the volume or raises the pressure in a system with a constant temperature. Thermal equilibrium is preserved by the procedure.
To know more about Isothermal visit:
https://brainly.com/question/12023162
#SPJ1
Which best represents the number of moles in exactly 108 grams of gold?
Answers
Answer: 0.548 mol
Explanation:
DOES ANYONE KNOW ANY OF THE ANSWERS TO THIS ??

Answers
Answer:
Which one do you need the answer to?
Explanation:
Consider the reaction
N2 + 3 H2 → 2 NH3 .
How much NH3 can be produced from the reaction of 74.2 g of N2 and 14.0 moles of H2?
Answers
The mass of ammonia that can be produced from the reaction of 74.2 g of N₂ and 14.0 moles of H₂ is 90.1 g.
What is the mole ratio of the reaction for the production of ammonia?The mole ratio of the reaction for the production of ammonia is obtained from the balanced equation of the reaction as follows:
Equation of the reaction: N₂ (g) + 3 H₂ (g) → 2 NH₃ (g)
The mole ratio of the reactants nitrogen and hydrogen is 1 : 3
Moles of nitrogen in 74.2 g of nitrogen = 74.2/28
Moles of nitrogen in 74.2 g of nitrogen = 2.65 moles
Moles of hydrogen present = 14.0 moles
The limiting reactant is nitrogen
Mole ratio of nitrogen to ammonia is 1 : 2
Mass of ammonia produced = 2.65 * 2 * 17 g
Mass of ammonia produced = 90.1 g
Learn more about mole ratio at: https://brainly.com/question/19099163
#SPJ1
energy extraction and the complete oxidation of foodstuffs requires three stages. the energy extracted from fuels is converted to atp. select the statements that are true for the different stages required for energy extraction and complete oxidation of foodstuffs. in the first and second stages the majority of atpatp needed for cellular processes is produced. in the first stag
Answers
The energy extracted from fuels is converted to ATP. in the first and second stages, the majority of the app needed for cellular processes is produced. in the first stage: True statement, in the third stage, is the fuel molecule.
Adenosine triphosphate (ATP) is the supply of strength for use and garage on the mobile degree. The structure of ATP is a nucleoside triphosphate, along with a nitrogenous base (adenine), a ribose sugar, and three serially bonded phosphate agencies.
ATP is able to power mobile strategies by shifting a phosphate group to some other molecule (a process referred to as phosphorylation). This transfer is done by way of special enzymes that couple the release of electricity from ATP to mobile activities that require energy.
Learn more about ATP here:
https://brainly.com/question/29551742
#SPJ4
The statements that are true for the different stages required for energy extraction and complete oxidation of foodstuffs are options D, e, and C.
An oxidation reaction occurs. Oxidation also can be described as the procedure of the elimination of hydrogen from the reactant species. Oxidation is the process of losing electrons by using a molecule, atom, or ion.
The oxidation of magnesium entails the chemical response between magnesium metallic and oxygen to shape magnesium oxide. Redox is a kind of chemical reaction wherein the oxidation states of substrate trade. Oxidation is the loss of electrons or a boom inside the oxidation country, likewise, the discount is the gain of electrons or a lower inside the oxidation nation.
Learn more about oxidation here:-https://brainly.com/question/157342
#SPJ4
Disclaimer:- your question is incomplete, please see below for the complete question.
Energy extraction and the complete oxidation of foodstuffs requires three stages. The energy extracted from fuels is converted to ATP.
Select the statements that are true for the different stages required for energy extraction and complete oxidation of foodstuffs. In the first and second stages the majority of ATP needed for cellular processes is produced.
a. In the third stage, fuel molecules are completely oxidized to CO2, and a small amount of ATP is produced.
b. In the third stage, fuel molecules are completely oxidized to CO2, and most of the ATP needed for cellular processes is produced.
c. In the third stage, fuel molecules are completely oxidized to CO2, and no ATP is produced.
d. In the first stage, macromolecules are converted to monomers, and a small amount of ATP is produced.
e. In the second stage, monomers are broken down, and a small amount of ATP is produced.
how does the polarity of a water molecule affect the interaction of water molecules with each other, or with other types of molecules?
Answers
The polarity of the water molecule decides whether a molecule will be soluble in water or not.
If we want a solute particle to be soluble in the solvent then it should match with the polarity of the solvent particles. In the case of water, the water is a polar molecule.
It means that whenever a polar molecule interacts with the molecular water it will immediately dissociate into water and will form a soluble compound.
But if a substance that is nonpolar in nature is dissolved in it the volatile solution in the water will be found on which a layer of the solute particle will be formed.
To know more about Polarity, visit,
https://brainly.com/question/17118815
#SPJ4
Cocoa beans are subjected to three processes during the manufacture of chocolate: cleaning, roasting, and 'nibbing'. Bags of cocoa beans are first cleaned, then cleaned beans are roasted, then roasted
Answers
Beans are processed through 'nibbing'. During the nibbing process, the roasted cocoa beans are crushed and ground into a paste called cocoa mass or cocoa liquor.
This cocoa mass can then be further processed to separate the cocoa solids from the cocoa butter, which is the fat component of the cocoa bean. The separated cocoa solids and cocoa butter are used in the production of chocolate. Pure cocoa mass (cocoa paste) in solid or semi-solid form is known as chocolate liquor. It includes about equal amounts of cocoa butter and solid cocoa, much like the cocoa beans (nibs) from which it is made. It is made from fermented, dried, roasted, and separated from their skins cocoa beans. To make cocoa mass (cocoa paste), the beans are pulverised.
To know more about cocoa liquor
https://brainly.com/question/5047676
#SPJ11
aspirin can be made in the laboratory by reacting acetic anhydride (c4h6o3) with salicylic acid (c7h6o3) to form aspirin (c9h8o4) and acetic acid (c2h4o2) . the balanced equation is
Answers
The balanced equation for the formation of aspirin is as follows:
\(C_7H_6O_3 + C_4H_6O_3\) → \(C_9H_8O_4 + C_2H_4O_2\)
The equation is balanced as there are equal numbers of atoms for each element on both sides.
The chemical formula of aspirin is \(C_9H_8O_4\) which is obtained by reacting acetic anhydride (\(C_4H_6O_3\)) with salicylic acid (\(C_7H_6O_3\)). The reaction also produces acetic acid (\(C_2H_4O_2\)) as a by-product.
The balanced chemical equation for the reaction is:
\(C_7H_6O_3 + C_4H_6O_3\) → \(C_9H_8O_4 + C_2H_4O_2\)
Salicylic acid is an organic acid that is found in various plants, including willow trees and is commonly used to treat pain and fever. Aspirin is a synthetic form of salicylic acid that is commonly used to treat pain and fever as well. It is one of the most widely used drugs in the world today.
Learn more about aspirin: https://brainly.com/question/25794846
#SPJ11
How to get rid of high cholesterol?!
Please tell me
Answers
you have to drink a lot of water and 67890
molecular formula CH- From among the 18 constitutional isomers of C His write structural formulas, and give the IUPAC names for those that are named as derivatives of (a) Heptane (b) Hexane (c) Pentane (d) Butane
Answers
Structural formula and IUPAC names of isomers of molecular formula CHAmong the 18 constitutional isomers of C, we have to write structural formulas and give the IUPAC names for those that are named as derivatives of (a) Heptane (b) Hexane (c) Pentane (d) Butane
.(a) Structural formulas and IUPAC names for isomers named as derivatives of Heptane are:Structural formula IUPAC name (1) CH3(CH2)5CH3 Heptane(2) CH3(CH2)4CH(CH3)CH3 2-Methylhexane(3) CH3(CH2)3C(CH3)2CH3 2,2-Dimethylpentane(4) CH3(CH2)2C(CH3)3 3,3-Dimethylpentane(5) CH3CH2C(CH3)2(CH2)3CH3 2,3-Dimethylpentane(6) CH3(CH2)2C(CH3)(CH2)4CH3 3-Ethylhexane(b) Structural formulas and IUPAC names for isomers named as derivatives of Hexane are:Structural formula IUPAC name (1) CH3(CH2)4CH3 Hexane(2) CH3(CH2)3CH(CH3)CH3 2-Methylpentane(3) CH3(CH2)2C(CH3)2CH3 2,3-Dimethylbutane(4) CH3CH(CH3)CH2(CH2)3CH3 3-Methylpentane(5) CH3(CH2)2C(CH3)(CH2)3CH3 2,3-Dimethylpentane
Structural formulas and IUPAC names for isomers named as derivatives of Pentane are:Structural formula IUPAC name (1) CH3(CH2)3CH3 Pentane(2) CH3(CH2)2CH(CH3)CH3 2-Methylbutane(3) CH3(CH2)CH(CH3)CH2CH3 2,3-Dimethylbutane(4) CH3C(CH3)2(CH2)2CH3 2,2-Dimethylbutane(5) CH3(CH2)2C(CH3)(CH2)2CH3 2,4-Dimethylpentane(6) CH3(CH2)2C(CH3)(CH2)CH(CH3)CH3 3,3-Dimethylpentane(7) CH3CH(CH3)CH2C(CH3)2CH3 2,3-Dimethylpentane(d) Structural formulas and IUPAC names for isomers named as derivatives of Butane are:Structural formula IUPAC name (1) CH3(CH2)2CH3 Butane(2) CH3CH(CH3)CH2CH3 2-Methylpropane (Isobutane).
To know more about IUPAC visit:
https://brainly.com/question/31327487
#SPJ11
When 6 g of granulated Zn is added to a solution of 2 M HCl in a beaker at room temperature, hydrogen gas is generated. How would the rate be affected if, at constant volume of the acid, the temperature is raised to 40C. The rate of hydrogen gas evolution will be unchanged. increased. decreased.
Answers
The rate of hydrogen gas evolution would increase if the temperature were raised to 40°C while maintaining the volume of acid constant.
When granulated zinc (6 g) is added to a solution of 2 M hydrochloric acid (HCl) at room temperature, hydrogen gas (H2) is produced. It's possible that the rate of hydrogen gas evolution would be affected if the temperature were raised to 40°C while keeping the volume of acid constant. The rate of hydrogen gas evolution would increase due to an increase in temperature causes the particles to move around more quickly, allowing more collisions to occur between zinc and hydrochloric acid particles. As a result, more hydrogen gas molecules are released from the acid.
Consequently, an increase in temperature has a positive effect on the reaction rate. According to the Arrhenius equation, the rate constant of a reaction increases when the temperature rises. The reaction rate is proportional to the rate constant. As a result, the reaction rate increases as the temperature increases. This implies that the rate of hydrogen gas evolution will rise when the temperature is raised to 40°C while keeping the volume of acid constant. As a result, the rate of hydrogen gas evolution would increase if the temperature were raised to 40°C while maintaining the volume of acid constant.
To know more about Arrhenius equation refer to:
https://brainly.in/question/12223513
#SPJ11
What is the density of water at room temperature in g/ml? give just the number and not the unit.
Answers
The density of water at room temperature is 1.0 g/ml.
Density is defined as the mass per unit volume. It gives the denseness of a given substance in a specific space. Different substances have different densities.
The formula to calculate the density is:
ρ =m/v
where,
ρ is the density, m is the mass of the object and V is the volume of the object. The SI unit of density is kg/m³, but we use g/ml for our convenience.
For example, the density of water is 1000 kg/m3 but we convert it into 1.0 g/ml.
If you need to learn more about density, click here
https://brainly.com/question/952755?referrer=searchResults
#spj4
Please Help!!!!!
7. If I initially have 4.0 L of a gas at a pressure of 1.1 atm, what will the volume be if I increase the pressure to 3.4 atm?
8. A toy balloon has an internal pressure of 1.05 atm and a volume of 5.0 L. If the temperature where the balloon is released is 200 C, what will happen to the volume when the balloon rises to an altitude where the pressure is 0.65 atm and the temperature is –150 C?
9. A small research submarine with a volume of 1.2 x 105 L has an internal pressure of 1.0 atm and an internal temperature of 150 C. If the submarine descends to a depth where the pressure is 150 atm and the temperature is 30 C, what will the volume of the gas inside be if the hull of the submarine breaks?
10. People who are angry sometimes say that they feel as if they’ll explode. If a calm person with a lung capacity of 3.5 liters and a body temperature of 360 C gets angry, what will the volume of the person’s lungs be if their temperature rises to 390 C. Based on this, do you think it’s likely they will explode?
Answers
Answer:
7. 1.29L
8. 4.29L
9. 855L
10. 3.53L
Explanation:


Which of the following cannot be used to show diffusion?
Select one:
a. A drop of food coloring added to water.
b. Moths attracted to a flame.
c. Students leaving school at the end of the day.
d. The spreading of the gases from a car's exhaust.
Answers
Moths being attracted to a flame cannot be used as an analogy for diffusion.
DiffusionIt is the movement of molecules from the region of higher concentration of the molecule to the region of lower concentration of the same molecule.
A drop of food coloring added to water will diffuse out until the entire water is covered by the coloring.
Students leaving school at the end of the day can be likened to diffusion because they move from regions of concentration to regions of lower concentration.
The spreading of exhaust gases is a diffusion.
More on diffusion can be found here: https://brainly.com/question/24746577
Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. ionic compound cation anion . + NaCl Na Cl Х $ ? Crci 6+ Cr CI Cro2 0 NHI 0 Ni(NO), I
Answers
An ionic compound's name and formula always place the cation before the anion.
Cations and anions in ionic compounds can be located in what ways?A cation's name, symbol, and charge must be noted in order to determine the ionic compound's formula. Determine the anion's identity after which you should note its symbol and charge. Create an electrically neutral substance by combining the two ions.In the formula for an ionic compound, the negative ion, known as an anion, is listed after the positive ion, known as a cation. The electrical charge in a balanced formula is zero, creating a neutral electrical charge.An ionic compound's name and formula always place the cation before the anion.To learn more about ionic compound's refer to:
https://brainly.com/question/2687188
#SPJ4
How would I go about doing this?
839 kJ/mol C ≡ C # of bonds ______ (product / reactant) side Energy:_____ 495 kJ/mo O = O # of bonds ______ (product / reactant) side Energy:_____ 413 kJ/mo C—H # of bonds ______ (product / reactant) side Energy:_____ 358 kJ/mo C—O # of bonds ______ (product / reactant) side Energy:_____ 467 kJ/mo H—O # of bonds ______ (product / reactant) side Energy:_____
Add the energies on the reactant side __________ kJ/mol
Add the energies on the product side __________ kJ/mol
This reaction is (exothermic / endothermic)
The reaction (produces / absorbs) __________ kJ when ____ moles of acetylene (C2H2) reacts.
Answers
Based on the values of bond energies given, the reaction is endothermic and absorbs 20 kJ of heat when 2 moles of acetylene reacts.
What is the energy change in the combustion of acetylene, C2H2?The equation of the combustion of acetylene sis given below:
2 C2H2 + 5 O2 -----> 4 CO2 + 2 H2O
Heat change, ΔH = Sum ΔH of bonds broken - sum of ΔH of bonds formed.Sum of ΔH bonds broken:
2 × C≡C + 2(2 × C—H) = 2 × 839 + 4 × 413 = 3330 kJ
5 × (2 × O = O) = 10 × 495 = 4950 kJ
Sum of ΔH bonds broken = 8280 kJ
Sum of ΔH of bonds formed:
Energy of C=O is 799 kJ
4 × 2 × C=O = 8 × 799 = 6392 kJ
Energy of H-O bond is 467kJ
2 × 2 × H-O = 2 × 2 × 467 = 1868 kJ
Sum of ΔH of bonds formed = 8260 kJ
Heat change, ΔH = 8280 -8260 kJ
Heat change, ΔH = 20 kJ
Therefore, based on the bond energies given, the reaction is endothermic and absorbs 20 kJ of heat when 2 moles of acetylene reacts.
Learn more about about heat change at: https://brainly.com/question/25109613
1) For the precipitation reaction of calcium oxalate below, the Ks = 3.7x10 Note: For this question, do not apply the small x approximation. A) If excess calcium oxalate were added to 100.0 mL of pure water, what concentration of calcium ions and oxalate ions would be expected when the solution is saturated? B) If 1.00 mg of calcium chloride were then added to the mixture (assume no solution volume change and complete dissolution and dissociation of CaCl2), what would be the expected concentrations of calcium ions and oxalate ions once equilibrium is reestablished? Ca2+(aq) + C2042(aq) ⇄ CaC204(s)
Answers
A) In precipitation reaction when the solution is saturated, the expected concentration of both calcium ions and oxalate ions would be approximately 0.0192 mol/L.
B) After equilibrium is reestablished, the expected concentrations of calcium ions and oxalate ions are approximately 2.498 × 10⁻⁴ mol/L and 0.0192 mol/L, respectively.
To answer the given questions about the precipitation reaction of calcium oxalate, let's break it down into two parts:
A) The concentration of calcium ions and oxalate ions in the saturated solution can be determined when an excess amount of calcium oxalate is added to 100.0 mL of pure water.
Since excess calcium oxalate is added, it means that the solution will contain more calcium oxalate than what can dissolve. At saturation, the solution is in equilibrium with the solid calcium oxalate.
Let's assume the concentration of calcium ions and oxalate ions in the saturated solution is represented by "x" (in mol/L).
The equilibrium expression for the reaction is:
Ks = [Ca²⁺][C₂O₄²⁻]
Given that the equilibrium constant Ks = 3.7 × 10⁻⁴, we can set up the equation:
3.7 × 10⁻⁴ = x * x
Solving for "x," we find:
x = √(3.7 × 10⁻⁴) ≈ 0.0192 mol/L
Therefore, when the solution is saturated, the expected concentration of both calcium ions and oxalate ions would be approximately 0.0192 mol/L.
B) If 1.00 mg of calcium chloride (CaCl2) were added to the mixture, what would be the expected concentrations of calcium ions and oxalate ions once equilibrium is reestablished?
Since calcium chloride (CaCl₂) dissociates completely into calcium ions (Ca²⁺) and chloride ions (Cl⁻) in solution, the addition of 1.00 mg of CaCl₂ will result in the addition of 1.00 mg of calcium ions.
First, we need to convert the mass of calcium ions from mg to mol:
1.00 mg = 0.001 g
0.001 g / (molar mass of Ca²⁺) = 0.001 g / 40.08 g/mol ≈ 2.498 × 10⁻⁵ mol
Since the solution volume is assumed to be unchanged, the concentrations of calcium ions and oxalate ions will change but not the molar amounts.
The concentration of calcium ions is the molar amount (2.498 × 10^(-5) mol) divided by the total solution volume (100.0 mL or 0.100 L):
Concentration of calcium ions = (2.498 × 10⁻⁵ mol) / 0.100 L ≈ 2.498 × 10⁻⁴ mol/L
The concentration of oxalate ions remains the same as in part A since the addition of calcium chloride does not affect the concentration of oxalate ions.
Therefore, after equilibrium is reestablished, the expected concentrations of calcium ions and oxalate ions are approximately 2.498 × 10⁻⁴ mol/L and 0.0192 mol/L, respectively.
Learn more about precipitation reaction at: https://brainly.com/question/13016165
#SPJ11
Banana slugs live on the ground in moist forests of the Pacific Northwest. They consume and break down a variety of materials, including small dead animals and rotting leaves. Which term describes banana slugs?
Group of answer choices
carnivores
consumers
decomposers
producers
PLEASE Answer This!!!
Answers
1.68g of phosphorus were burned to form 3.87g of phosphorus oxide. Calculate its empirical formula
Answers
1.68g of phosphorus were burned to form 3.87g of phosphorus oxide, Then the empirical formula is P₂O₅.
Molecular formula = P₄O₆
Mass of one mole of oxide Molar mass = 4 * 31 + 6 * 16
= 124 + 96
= 220 g/mol
Molar mass of Phosphorus = 31 g/mol
Molar mass of Oxygen = 16g/mol
wp = 1.68g
Moles(Phosphorus) = 1.68 / 31 = 0.054
wo = 3.87 - 1.68
= 2.19
Moles(Oxygen) = 2.19/16
= 0.138
P : O
0.054 : 0.1368
0.054/0.054 : 0.1368/0.054
1 : 2.5
1 * 2 : 2.5*3
2 : 5
Emperical formula : P₂O₅
To know more about Emperical formula check the below link:
https://brainly.com/question/28116165
#SPJ1
Are the following chemical equations reversible or irreversible?
2H2O ←→ H3O+ + OH-
HA + H2O ←→ A- + H3O+
HA + H2O → A- + H3O+
MOH → M+ + OH-
Answers
The first two chemical equations are reversible while the other two are irreversible.
What are chemical equations?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equations,here:
https://brainly.com/question/19626681
#SPJ1
if , group of answer choices the activation energy will change as the reaction progresses. the forward reaction will have a greater activation energy than the reverse reaction. the forward reaction is slower than the reverse reaction. the collision energy of the reactants will be greater than that of the products. the reaction rate will speed up with time.
Answers
The activation energy refers to the amount of energy required for a chemical reaction to occur. As the reaction progresses, the activation energy can change depending on the specific reaction conditions.
In some cases, the forward reaction may have a greater activation energy than the reverse reaction, which means that it will require more energy to proceed. Additionally, the forward reaction may be slower than the reverse reaction due to the higher activation energy barrier. This is because the collision energy of the reactants will be greater than that of the products, which makes it more difficult for the reaction to proceed in the forward direction.
However, over time, the reaction rate will speed up as more and more reactants collide and overcome the activation energy barrier. This increase in speed will eventually lead to a state of equilibrium where the forward and reverse reactions occur at equal rates. If the collision energy is greater than the activation energy, the reaction rate will speed up with time as more reactant molecules are converted into products.
Learn more about activation energy here:
brainly.com/question/10507976
#SPJ11
fluorine electron configuration
Answers
Answer:
what about it
Explanation:
Chemists can identify the composition of some unknown salts by conducting a flame test. When potassium salts are heated in a flame, a purple color is observed.
This is due to the movement of electrons between energy levels. What is the electron configuration of a potassium atom at ground state?
answer choices
1s2; 2s2; 2p6; 3s2; 3p6; 4d1
1s2; 2s2; 2p6; 3s2;3p6; 3d1
1s2; 2s2; 2d6; 3s2; 3d6; 4s1
1s2; 2s2; 2p6; 3s2; 3p6; 4s1
Answers
A potassium atom's ground state electron configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s1.
What substance is electronic configuration 1s2 2s2 2p6 3s2 3p6 4s1?An atom's electron configuration is a picture of how electrons are arranged in relation to orbital shells and subshells. Consequently, this is potassium's electron configuration.
How can you express a whole electron configuration in writing?Making Electron Configurations in Writing. Write the energy level (the period) first, then the subshell that needs to be filled, and finally the superscript, which indicates how many electrons are in that subshell. The atomic number, Z, is the sum of all the electrons.
To know more about electron configuration visit:-
https://brainly.com/question/29757010
#SPJ4
How many electrons are transferred from glyceraldeyde 3 phosphate to nad in glycolysis?
Answers
Electrons transferred from Glyceraldehyde 3 phosphate to NAD in glycolysis per glucose are 4.
What are electrons?The electron is a subatomic particle (denoted by symbol e−or β−) whose electric charge is negative one elementary charge. Electrons belong to first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1836 times smaller than that of proton.
Quantum mechanical properties of electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both the particles and waves: They can collide with the other particles and can be diffracted like light. The wave properties of electrons are easier to observe with the experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for given energy.
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry and thermal conductivity, and they also participate in the gravitational, electromagnetic and weak interactions. Since an electron has charge, it has a surrounding the electric field, and if that electron is moving relative to an observer, said observer will observe it to generate a magnetic field. Electromagnetic fields produced from other sources will affect the motion of an electron according to Lorentz force law.
Electrons radiate or absorb energy in form of photons when they are accelerated. Laboratory instruments are capable of trapping individual electrons as well as electron plasma by use of electromagnetic fields. Special telescopes can detect the electron plasma in outer space. Electrons are involved in many applications such as the tribology or frictional charging, electrolysis, electrochemistry, battery technologies, electronics, welding, cathode-ray tubes, photoelectricity, photovoltaic solar panels, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
To know more about electrons visit: brainly.com/question/1255220
#SPJ4
A dehydration reaction is the process in which __________.
Answers
Answer:
Dehydration reactions can be defined as the chemical reactions in which a water molecule is eliminated from the reactant molecule. The process of combination of two molecules with the elimination of water molecule is called dehydration synthesis.