he standard enthalpy of combustion of cyclopropane, c3h6, is −2091 kj mol−1 at 298 k. calculate the standard enthalpy of formation of cyclopropane at 298 k (20 marks) (ii) the enthalpy of formation of propene, ch3ch
Answers
The standard enthalpy of formation of cyclopropane at 298 K is -2555 kJ/mol.
The standard enthalpy of formation (∆Hf) is the change in enthalpy that occurs when one mole of a compound is formed from its constituent elements in their standard states. To calculate the standard enthalpy of formation of cyclopropane (C3H6) at 298 K, we can use the following equation:
∆Hf = ∆Hcombustion of C3H6 - [∆Hcombustion of C + 3∆Hcombustion of H2]
Given that the standard enthalpy of combustion (∆Hcombustion) of cyclopropane is -2091 kJ/mol, we need to know the standard enthalpies of combustion for carbon (∆Hcombustion of C) and hydrogen (∆Hcombustion of H2).
Assuming carbon and hydrogen are in their standard states, we can use the following values:
∆Hcombustion of C = -394 kJ/mol
∆Hcombustion of H2 = -286 kJ/mol
Plugging these values into the equation:
∆Hf = -2091 kJ/mol - [(-394 kJ/mol) + 3(-286 kJ/mol)]
= -2091 kJ/mol - (-394 kJ/mol - 858 kJ/mol)
= -2091 kJ/mol - 464 kJ/mol
= -2555 kJ/mol
Therefore, the standard enthalpy of formation of cyclopropane at 298 K is -2555 kJ/mol.
Learn more about enthalpy from given link: https://brainly.com/question/16720480
#SPJ11
Related Questions
Correct question to answer : Wine goes bad soon after opening because the ethanol CH3CH2OH dissolved in it reacts with oxygen O2 gas to form water and aqueous acetic acid CH3COOH, the main ingredient in vinegar. Calculate the moles of ethanol needed to produce 0.900mol of acetic acid. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits.Do not answer : Ammonium phosphate ((NH4)3(PO4) is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid with liquid ammonia. Calculate the moles of ammonia needed to produce of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to significant digits.
Answers
• The balanced equation for the reaction is given by:
CH3CH2OH + O2 → CH3COOH+ H2O• From the above reaction we can see that:
1 mole of ethanol (CH3Ch2OH) produces 1 mol of acetic acid(CH3COOH)
so . x mole of ethanol will produce 0.9mol of acetic acid ....(cross multiply)
xmol ethanol * 1mol acetic = 1mol ethamol* 0.9molacetic
∴ xmol ethanol = 1*0.9 /1
= 0.90 mol
• This means that, 0.90 mol of ethanol, is needed to produce 0.9mol acetic acid,.
water has a higher surface tension than most liquids because of ____________ bonds its molecules form
Answers
Water has a higher surface tension than most liquids because of hydrogen bonds its molecules form.
Water has a higher surface tension than other liquids due to the relatively high molecular or strong cohesive interactions that occur between its molecules. Hydrogen bonds also make it possible for water molecules to strongly adhere to one another and resist stretching.
The formed links are known as hydrogen bonds, and they cause the water molecules to stick together tightly and have a high surface tension.
A robust and flexible lattice of water molecules is created when several water molecules form hydrogen bonds with one another. High surface tension results from this. Water striders may move across the water's surface thanks to surface tension.
To know more about surface tension, visit,
https://brainly.com/question/138724
#SPJ4
Which statement about an atom is correct?(1 point)
The electron has a negative charge and is found outside of the nucleus.
The electron has a negative charge and is found outside of the nucleus.
The neutron has a negative charge and is found in the nucleus.
The neutron has a negative charge and is found in the nucleus.
The proton has no charge and is found in the nucleus.
The proton has no charge and is found in the nucleus.
The neutron has no charge and is found outside of the nucleus.
Answers
Answer:
the electron has a negative charge and is found outside the nucleus
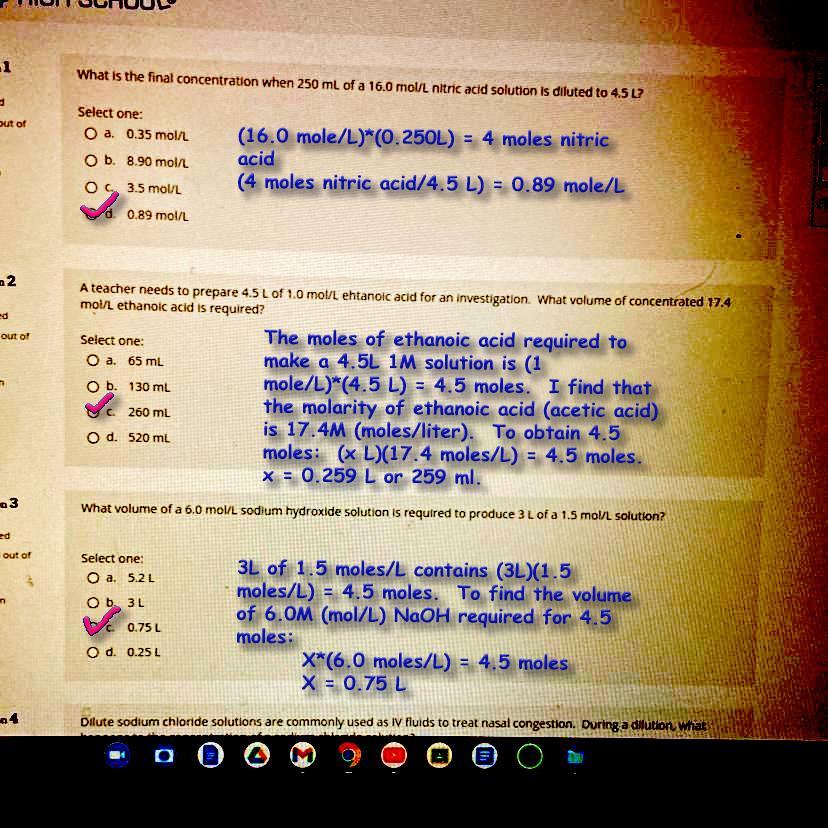
see the attached photo*** can someone please explain how to do this for me? I really need help on my summer school thank you !

Answers
Answer:
See attached image.
Explanation:
See attached image.
Remember to use the units in deciding steps to take. Find the moles needed or delivered by using:
Molarity x Volume = moles [(moles/L)*(L) = moles]
Molarity, M, is dedined as 1 mole/liter. When working with a unit such as 6M, rewite it as 6 moles/liter, and then use unit conversions to guide how to find the solution, so to speak.
In terms of carbon monoxide, 1 marijuana joint is the equivalent of how many cigarettes?.
Answers
The main chemical compound in marijuana that has a harmful effect on cells lining the airways is tetrahydrocannabinol, or THC. This compound is similar to nicotine, which is the primary harmful chemical compound in cigarette smoke.
In addition to THC, marijuana smoke also contains more tar than cigarette smoke. Tar is made up of small particles that are easily inhaled and are known to be carcinogenic. The combination of the THC, nicotine, and tar in marijuana smoke is what makes it so damaging to cells lining the airways.
The daily smoking of one marijuana joint is equivalent to the amount of damage to cells lining the airways as around five cigarettes.
To know more about cigarettes, visit;
https://brainly.com/question/30902635
#SPJ1
FILL IN THE BLANK. ___ occurs when an electron in an atom jumps from a lower energy orbital to a higher energy orbital.
Answers
Radiation occurs when an electron in an atom jumps from a lower energy orbital to a higher energy orbital.
Define electrons.
The elementary electric charge of the electron is a negative one, making it a subatomic particle. Due to their lack of known components or substructure, electrons, which are part of the first generation of the lepton particle family, are typically regarded to be elementary particles.
Energy released by matter as rays or swift particles is known as radiation. Atoms make up all physical matter. The nucleus of an atom includes tiny particles called protons and neutrons, and the outer shell of the atom is made up of other particles called electrons.
An electron loses a significant portion of its energy through a radiative nuclear interaction at extremely high speeds.
To learn more about radiation use:
https://brainly.com/question/31285748
#SPJ4
The solute is designated as the dispersed phase in a colloidal suspension.
A. true
B. false
Answers
Answer: Im not, sure if this is right, but I personally think that the Answer, to your question is TRUE!
Explanation: But Dont just take my word for it as I am not sure, Myself. I only made a guess.
What is the function of each of these structures?
Answers
what is the name of the second halogen
Answers
Answer:
flourine
Explanation:
im not sure the answer
A graph of gas pressure versus the number of particles in a container is a straight line. Which other relationship will have a similar graph? volume versus pressure, because they are also directly proportional volume versus temperature, because they are also directly proportional volume versus pressure, because they are also inversely proportional volume versus temperature, because they are also inversely proportional
Answers
Answer:
b
Explanation:
gitcy is good for your spleen
The other relationship that will have a similar graph is volume versus temperature, because they are also directly proportional according to Charles's Law.
What is Charles's Law?Charles's Law is a gas law that states that the volume of a fixed mass of gas at a constant pressure is directly proportional to its absolute temperature.
This means that as the temperature of a gas increases, the volume of the gas will also increase proportionally, and as the temperature of a gas decreases, the volume of the gas will decrease proportionally.
Therefore, if a graph of gas pressure versus the number of particles in a container is a straight line, a graph of volume versus temperature will have a similar shape and be directly proportional, because they are both affected by changes in temperature.
Learn more about Charles's Law at:
https://brainly.com/question/16927784
#SPJ7
an herbicide contains only c , h , cl , and n . the complete combustion of a 150.0 mg sample of the herbicide in excess oxygen produced 156.9 ml of co2 and 91.52 ml of h2o vapor at stp. a separate analysis determined the 150.0 mg sample contained 41.36 mg cl . determine the percent composition of the herbicide.
Answers
The percent composition of the herbicide is 44.5% C, 6.27% H, 22.9% Cl, and 26.4% N.
To solve this problem, we will use the information provided to calculate the percent composition of the herbicide.
First, let's calculate the number of moles of CO2 and H2O produced by the combustion of the herbicide. We can use the ideal gas law to do this:
n_CO2 = (156.9 mL) / (22.4 L/mol) * (1 mol CO2 / 1 L) = 7.00 mol CO2
n_H2O = (91.52 mL) / (22.4 L/mol) * (1 mol H2O / 1 L) = 4.08 mol H2O
Next, let's calculate the number of moles of carbon, hydrogen, and nitrogen in the herbicide using the combustion reaction:
C_xH_yCl_zN_w + (x + y/4 - z/2) O2 → x CO2 + (y/2) H2O + z HCl + w NO2
From the balanced equation, we can see that the number of moles of CO2 produced is equal to the number of moles of carbon in the herbicide, and the number of moles of H2O produced is equal to the number of moles of hydrogen in the herbicide.
We can use this information to solve for the number of moles of carbon, hydrogen, and nitrogen in the herbicide:
n_C = 7.00 mol CO2
n_H = 8.16 mol H2O
n_Cl = 41.36 mg / 35.45 g/mol / 0.1500 g = 0.767 mol Cl
Since the herbicide contains no other elements besides C, H, Cl, and N, we can assume that the mass of the herbicide is equal to the sum of the masses of these elements. We can use this information to solve for the mass of the herbicide:
m_Herbicide = m_C + m_H + m_Cl + m_N
m_Herbicide = n_C * 12.01 g/mol + n_H * 1.008 g/mol + n_Cl * 35.45 g/mol + n_N * 14.01 g/mol
We can rearrange this equation to solve for the percent composition of the herbicide:
% C = (n_C * 12.01 g/mol / m_Herbicide) * 100% = 44.5%
% H = (n_H * 1.008 g/mol / m_Herbicide) * 100% = 6.27%
% Cl = (n_Cl * 35.45 g/mol / m_Herbicide) * 100% = 22.9%
% N = ((m_Herbicide - n_C * 12.01 g/mol - n_H * 1.008 g/mol - n_Cl * 35.45 g/mol) / m_Herbicide) * 100% = 26.4%
to know more about herbicides refer here:
https://brainly.com/question/31375814#
#SPJ11
Avogadro's number is equal to?
Answers
Answer:
6.273×10²³
Explanation:
hope this is useful friend
Answer:
6.023 × 10²³
Explanation:
Avogadro's number is used for calculating masses and moles and can applied to Stoichiometry. Avogadro's number is useful for atoms and molecules
a 3.79 l container can hold 0.15 moles h2 gas. what mass of zn is required to generate 0.15 mol h2
Zn + 2HCl -> ZnCl2 + H2
Answers
The mass of zinc, Zn required to generate 0.15 mole of H₂, given the reaction is 9.807 grams
How do I determine the mass of Zn required?Let us consider the balanced equation to obtain useful information.
Zn + 2HCl -> ZnCl₂ + H₂
From the balanced equation above,
1 mole of H₂ was obtained from 1 mole of Zn
Therefore,
0.15 mole of H₂ will also be obtained from 0.15 mole of Zn.
Finally, we can obtain the mass of zinc, Zn required as follow:
Molar mass of zinc, Zn = 65.38 g/mol Mole of zinc, Zn = 0.15 moleMass of zinc, Zn = ?Mole = mass / molar mass
1.15 = Mass of zinc, Zn / 65.38
Cross multiply
Mass of zinc, Zn = 0.15 × 65.38
Mass of zinc, Zn = 9.807 grams
Thus, the mass of zinc, Zn required is 9.807 grams
Learn more about mass:
https://brainly.com/question/21940152
#SPJ1
Next to a shallow cylindrical lake with a radius of 4km and an average water height of 5m, a type A exhaust basin has been installed, which recorded a total water loss of 4.5cm during a summer month. It is requested to calculate the evaporation of the lake and the volume of the lake water in cubic meters for the specific time period if the coefficient of the evaporation basin is equal to 0.7
Answers
In a shallow cylindrical lake with a radius of 4 km and an average water height of 5 m, a type A exhaust basin recorded a total water loss of 4.5 cm during a summer month.
The task is to calculate the evaporation of the lake and the volume of lake water in cubic meters for that specific time period, assuming an evaporation coefficient of 0.7. To calculate the evaporation of the lake, we first convert the recorded water loss from centimeters to meters. The total water loss is 4.5 cm, which is equal to 0.045 meters.
The evaporation from the lake can be determined by multiplying the water loss by the evaporation coefficient. In this case, the evaporation coefficient is given as 0.7. So, the evaporation from the lake is calculated as:
Evaporation = Water loss * Evaporation coefficient
Evaporation = 0.045 m * 0.7 = 0.0315 m
Therefore, the evaporation of the lake during the specified time period is 0.0315 cubic meters.To calculate the volume of lake water, we need to consider the shape of the lake, which is a shallow cylinder. The formula for the volume of a cylinder is:
Volume = π * radius^2 * height
Given that the radius of the lake is 4 km (4000 m) and the average water height is 5 m, we can calculate the volume of the lake as:
Volume = π * (4000 m)^2 * 5 m = 251,327,412 m^3
Therefore, the volume of lake water for the specific time period is approximately 251,327,412 cubic meters.
Learn more about evaporation here:- brainly.com/question/28319650
#SPJ11
Can someone help me thank you. I have to look at each picture and determine if the circuits shown are series circuits or parallel circuits. Explain how you know.

Answers
Answer:
I believe it may be-
1. Series
2. Parallel
3. Parallel
4. Series
Explanation:
In a series circuit, electricity only has one path to follow while a parallel circuit has more than one path to follow.
What is the heaviest element that was created in the Big Bang?
Hint: The early universe remained hot enough for fusion for only a short time.
a. Deuterium
b. Magnesium
c. Lithium
d. Iron
Answers
The heaviest element that was created in the Big Bang is lithium (option c).
During the early stages of the universe, known as Big Bang nucleosynthesis, the conditions were hot and dense enough for the fusion of protons and neutrons to form light elements. While hydrogen and helium were the most abundant elements produced, a small amount of lithium-7 was also synthesized. However, the production of heavier elements through fusion processes required the later formation of stars and subsequent stellar nucleosynthesis.
Hence, the correct option is option c.
Learn more about the big bang here:
https://brainly.com/question/17209127
#SPJ 4
How many moles are in 0.0688 g of silver chloride (AgCl)
Answers
Answer: 0.00048
m
o
l
Explanation:
Moles = Mass/Molar mass
n
=
m
M
r
=
0.0688
107.87
+
35.5
=
0.00048
m
o
l
Click this link to view O*NET's Work Context section for Emergency Medical Technicians. Note that common tools
are listed toward the middle. According to O*NET, what are some common work tools for Emergency Medical
Technicians? Select four options.
ladders, scaffolds, or poles
Airway suction units
Intravenous IV pressure infusers;
Head and body pumps
Cardboard splints
Full-spine immobilization devices
Answers
Answer:
( B, C, E, F)
Explanation:
Person above is right!
But I hope this helps!!
Intravenous IV pressure infusers, Airway suction units, full spine immobilization devices, and cardboard splints are required for Emergency Medical Technicians.
What are medical technicians?A medical worker known as a medical technician supports doctors and hospitals and hence plays a crucial role in the healthcare sector. They have various important roles during medical emergencies.
They are in charge of duties like checking blood in labs or physiological fluids like perspiration or tissue samples so doctors can decide on treatment options in a well-informed manner.
Intravenous IV pressure infusers, Airway suction units, full spine immobilization devices, and cardboard splints units are required for Emergency Medical Technicians. Hence, options B, C, E, and F are correct.
Learn more about medical technicians, here:
https://brainly.com/question/30300094
#SPJ5
Speed is the rate of change of position expressed as _____ traveled per unit of time. A. direction B. meter C. displacement D. distance
Answers
Answer. Speed is the rate of change of position expressed as distance travelled per unit of time
give brainliest if right
Answer:
distance
Explanation:
When solutions of barium chloride (aq) and sodium sulfate (aq) are mixed, what is/are the spectator ion(s)?
Answers
When solution aqueous solutions of Barium chloride and Sodium sulphate are mixed, here Sodium and Chloride ions are spectator ions as their state does not change in this reaction.
Spectator ions in any reactions are the ions who does not participate in the particular reaction. The state of these ions is same throughout the reaction. The charges on these ions remains same they do not get oxidized or reduced. Spectator ions do not get precipitate in any reaction. They can be cancelled from both side of the reaction. When aqueous solutions of Barium chloride and Sodium sulphate reacts, the resultant product will be Barium chloride and Sodium sulphate. It is an example of double displacement reaction.
Learn more about spectator ions here:
https://brainly.com/question/28913274
#SPJ4

Does someone know the answers?

Answers
Answer:
b)sugar and water : Sedementation
d) salt and water : evaporation
Write the empirical formula for at least four ionic compounds that could be formed from the following ions:

Answers
Answer:
Fe(C₂H₃O₂)₃, Fe(CN)₃, Pb(C₂H₃O₂)₄, Pb(CN)₄
Explanation:
Cations (positively charged ions) can only form ionic bonds with anions (negatively charged ions). However, you can't just simply put one cation and one anion together to form a compound. Each compound needs to been neutral, or have an overall charge of 0. When cations and anions do not have charges that perfectly cancel, you need to modify the amount of each ion in the compound.
1.) Fe(C₂H₃O₂)₃
-----> Fe³⁺ and C₂H₃O₂⁻
-----> +3 + (-1) + (-1) + (-1) = 0
2.) Fe(CN)₃
-----> Fe³⁺ and CN⁻
-----> +3 + (-1) + (-1) + (-1) = 0
3.) Pb(C₂H₃O₂)₄
-----> Pb⁴⁺ and C₂H₃O₂⁻
-----> +4 + (-1) + (-1) + (-1) + (-1) = 0
4.) Pb(CN)₄
-----> Pb⁴⁺ and CN⁻
-----> +4 + (-1) + (-1) + (-1) + (-1) = 0
What happens to
carbon dioxide?
Answers
PLEASEEEE HELP :')
''If 5.6 mole of calcium metal is reacted, how many grams of calcium phosphide will form?'
Answers
Answer:
340 grams Ca₃P₂ (2 sig. figs.)
Explanation:
3Ca + 2P => Ca₃P₂
5.6 mole + excess => ? grams
Convert the 'known' to a coefficient of 1 by dividing all coefficients by 3.
=> Ca + 2/3P => 1/3Ca₃P₂
From the above, 1 mole of Ca => 1/3 mole Ca₃P₂
∴ 5.6 mole Ca in an excess of P => 1/3(5.6 mole) Ca₃P₂
=> 1.8666 mol Ca₃P₂ (calculator answer) ≅ 1.9 mol Ca₃P₂
=> 1.9 mole x 182 g Ca₃P₂/mol Ca₃P₂ = 339.73333 grams Ca₃P₂
≅ 340 grams Ca₃P₂ (2 sig. figs.)
31.The following equation represents which of the following types of reaction?4 H3PO4 ---> P4 + 5 O2 + 6 H2OSelect one:a. Decomposition.b. Double replacement.c. Single replacement.d. Synthesis.
Answers
Answer
A. Decomposition.
Explanation
A decomposition reaction can be defined as a chemical reaction in which one reactant breaks down into two or more products.
The equilibrium constant Kc for the reaction
H2(g) + Br2(g) ⇆ 2HBr(g)
is 2.180 × 106 at 730°C. Starting with 5.20 moles of HBr in a 18.7−L reaction vessel, calculate the concentrations of H2, Br2, and HBr at equilibrium.
[H2] = ___M
[Br2]= ___ M
[HBr]= ___M
PLEASE I NEED ANSWER ASAP!
Answers
The equilibrium concentrations are approximately:
[H2] = 0 M
[Br2] = 0 M
[HBr] = 0.668 M.
What is equilibrium constant?At equilibrium, the equilibrium constant, K, indicates the connection between the reactants and products of a reaction. When the rates of forwarding and backward reactions are equal, a reaction has reached equilibrium.
The equilibrium constant expression for the reaction is:
Kc = [HBr]^2 / ([H2] * [Br2])
We are given that Kc = 2.180 × 10^6 at 730°C. We can use this information to set up an ICE (initial, change, equilibrium) table and solve for the equilibrium concentrations of the three species.
Initial concentrations:
[HBr] = 5.20 mol / 18.7 L = 0.278 M (since 2HBr are formed from 1 mol Br2 and 1 mol H2, initial [Br2] and [H2] are both 0)
Change in concentrations:
Let x be the change in concentration of HBr. Then, the change in concentration of H2 and Br2 will be -2x and -x, respectively.
Equilibrium concentrations:
[H2] = 0 - 2x
[Br2] = 0 - x
[HBr] = 0.278 + x
Substituting these concentrations into the equilibrium constant expression and solving for x gives:
Kc = [HBr]^2 / ([H2] * [Br2])
2.180 × 10^6 = (0.278 + x)^2 / (2x * x)
Solving for x using this equation gives:
x = 0.390 M
Therefore, the equilibrium concentrations are:
[H2] = 0 - 2x = -0.780 M (Note: negative concentration is unphysical, but indicates that all H2 reacted)
[Br2] = 0 - x = -0.390 M (Note: negative concentration is unphysical, but indicates that all Br2 reacted)
[HBr] = 0.278 + x = 0.668 M
To know more about equilibrium, visit:
https://brainly.com/question/30694482
#SPJ1
which molecules are used to produce energy
Answers
Answer:
Explanation:
fats, proteins, and carbohydrates
which ion is responsible for the solution being acidic or basic nac2h3o2
Answers
The ion responsible for the solution being basic or acidic in NaC2H3O2 is the Acetate ion.
The Acetate ion, CH3COO- is responsible for the solution being acidic or basic in NaC2H3O2.
NaC2H3O2 is also known as Sodium Acetate. It is a common compound in the laboratory that is colorless, deliquescent, and odorless. It dissolves easily in water, and its pH varies depending on the solution's acetate and acetic acid concentration.
Because acetate ion is a weak base, its solution has a higher pH than a solution containing just the acid. The buffer capacity of a solution of the salt NaC2H3O2 (Acetate ion) is dependent on the concentration of the salt and the pH of the solution. A solution with a pH of 7.0 and a 0.1 M NaC2H3O2 concentration would have a buffer capacity of 1.4. A solution with a pH of 5.0 and the same salt concentration would have a buffer capacity of 13.0.The equation for the dissociation of sodium acetate is given below:
NaC2H3O2 ⇌ Na+ + C2H3O2-
We can say that the solution is basic if the pH is greater than 7, acidic if the pH is less than 7, and neutral if the pH is equal to 7.
Hence, the Acetate ion is responsible for the solution being basic or acidic in NaC2H3O2.
learn more about dissociation here
https://brainly.com/question/16818822
#SPJ11
Carlita’s body is made up of many cells. What is one thing all her cells have in common?
Answers
Answer:
All of them have a nucleus..
Explanation:
Pretty simple stuff. There's no cell without one.
Carlita’s body is made up of many cells. The one thing that all her cells have in common is they have the same kind of genes.
What are genes?Genes are tiny pieces of spherical hereditary material that are bead-shaped and found on chromosomes. They hold the organism's genetic makeup. The number of genes on each chromosome is incalculable.
The hereditary material that makes up chromosomes is arranged in x shapes inside the nucleus. DNA, genes, and proteins make it up. An organism's genotype is distinct from other organisms because of its distinct genetic makeup, and this distinction can be passed down from one generation to the next.
Traits may be influenced by genes, the environment, or a mixture of the two. Quantitative features can also be qualitative, such as eye colour (such as height or blood pressure).
Therefore, numerous cells make up Carlita's body. Her cells all share the same type of genes, which is the only thing that unites them.
To learn more about genes, refer to the link:
https://brainly.com/question/8046559
#SPJ6
Explain why you need to use a clean pipette tip to transfer each DNA sample to the gel plate.
Answers
Answer: Sterilization
Explanation:
The pipette needs to be sterile so that nothing interferes with the DNA sample.