Answers
Answer:
your answer is
A spot of the mixture is placed near the bottom of a piece of chromatography paper. The paper is then placed upright in a suitable solvent , such as water. As the solvent soaks up the paper, it carries the mixtures with it. Different components of the mixture will move at different rates.
Hope it’s helps you Buddy
Explanation:
Related Questions
benzen has a boiling point of 80.10 c we know the change in boiling point for a solution of c6h14 in benzen is 2.25 what is the new boiling point for the solution
bp= ? c
Answers
The pressure of the environment affects the liquid's boiling point. The boiling point of the liquid is higher when it is under high pressure than when it is under normal atmospheric pressure. For a given pressure, various liquids have different boiling points.
The temperature at which a liquid's vapour pressure equals the surrounding atmosphere is known as the boiling point of the liquid. This temperature causes the liquid to become a vapour.
The temperature of the liquid, the pressure of the atmosphere, and the pressure of the vapour all affect its boiling point.
We know that change in temperature of a system is given by the following formula:
Initial boiling point (T₁) = 80.1 °C
ΔT = T₂ - T₁
2.25 = T₂ - 80.1
T₂ = 82.35 °C
To know more about boiling point, visit;
https://brainly.com/question/28986258
#SPJ1
Answer:
82.35
Explanation:
acellus
Express your answers as integers separated by commas.
Answers
Not enough information man
Help bestie pls help ASAP thank you
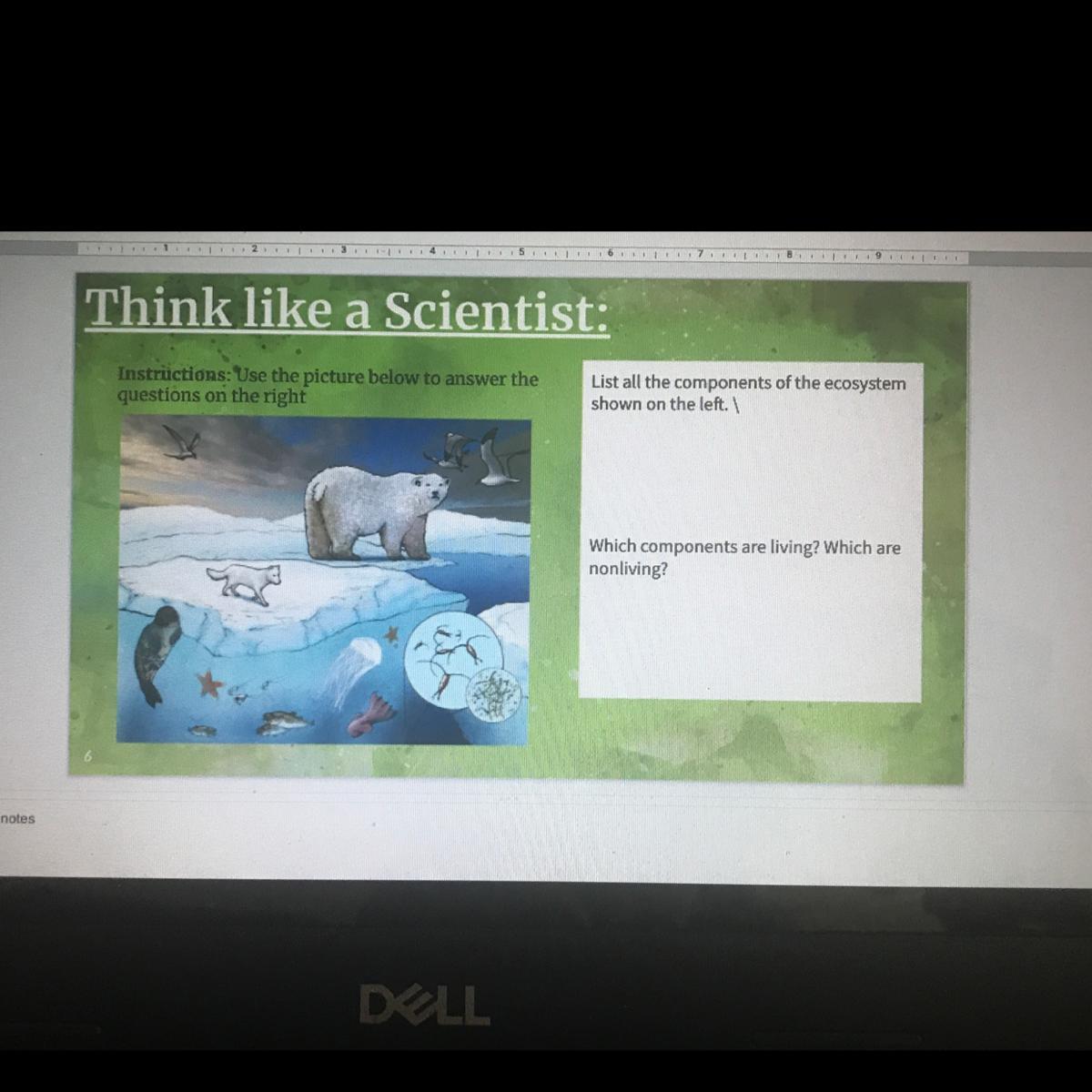
Answers
Explanation:
heyyyyyyyyy how are you sure
The surface particles of a sample of matter experiences a strong downward pull
and stretch tight enough for a spider to walk on. This is an example of?
HELP PLEASE ITS DUE TODAY, THANK U!
Answers
How many moles are there in 1.20 x 1025 particles of helium?
Answers
Answer:
You would get 19.969 moles.
Explanation:
You would get 19.969 moles.
The same mass of 5 different potential fuels was used to heat the same mass of water in a simple calorimeter. The results are shown below. Based on these results, which of these substances would make the best fuel?
Answers
We can see here that the best fuel is the one that produces the most heat per unit mass. In this case, the fuel that produces the most heat per unit mass is methanol.
What is fuel?Fuel is a substance that is used to produce energy through combustion or other chemical reactions. It is commonly utilized to power various forms of transportation, generate heat or electricity, and operate machinery and appliances.
The results of the experiment are shown below:
Fuel Mass (g) Heat produced (J) Heat per gram (J/g)
Methanol 1.0 350 350
Ethanol 1.0 250 250
Propane 1.0 200 200
Butane 1.0 150 150
Pentane 1.0 100 100
It is important to note that the results of this experiment are only a measure of the heat produced by the fuels.
Learn more about fuel on https://brainly.com/question/10172005
#SPJ1
what energy is stored
Answers
Answer: potential energy is stored
Explanation:
gas syringe
bung
chips
25 cm of dilute
hydrochloric acid
Which channes slow down the rate of reaction?
Decrease the size of pieces of marble chips
Decrease surface area of marble chips
Inercase concentration of acid
Increase temperature of acid
Answers
Answer:
Decrease surface area of marble chips.
Explanation:
Because the reaction goes on the surface of marble chips, decreasing surface area of marble chips will decrease(slow down) the rate of the reaction.
If a solute in water has a concentration of 0.12 mg/mL and has a concentration in ether of 0.60 mg/mL, what is the distribution coefficient, K, between ether and water
Answers
Answer:
K = 5
Explanation:
Distribution coefficient, K, is defined as the ratio between the concentration of a solute in two inmiscible solvents.
K = Concentration in solvent 1 / Concentration in solvent 2
Usually solvent 2 is water.
Replacing with the concentrations in the problem:
K = Concentration in ether / Concentration in water
K = 0.60mg/mL / 0.12mg/mL
K = 53. A Wilkinson’s catalyst is widely used in the hydrogenation of alkenes. Show a catalytic cycle, including: i. chemical structure of the catalyst, with complete stereochemistry ii. molecular geometry of catalyst iii. type of reactions involved iv. the appropriate starting material, reagent and solvent v. major and minor end-products vi. all intermediates, for each reaction stated in (iii)
Answers
We can see here that the catalytic cycle for the hydrogenation of alkenes using Wilkinson's catalyst involves several steps.
What are the steps involved?Here's an overview of the catalytic cycle, including the necessary details:
i. Chemical structure of the catalyst:
Wilkinson's catalyst, also known as chloridotris(triphenylphosphine)rhodium(I), has the following chemical structure: [RhCl(PPh3)3]
ii. Molecular geometry of the catalyst:
The Wilkinson's catalyst has a trigonal bipyramidal geometry around the rhodium center. The three triphenylphosphine (PPh3) ligands occupy equatorial positions, while the chloride (Cl) ligand occupies an axial position.
iii. Type of reactions involved:
The catalytic cycle involves several reactions, including:
Oxidative addition: The rhodium center undergoes oxidative addition, reacting with molecular hydrogen (H2) to form a dihydride intermediate.Alkene coordination: The alkene substrate coordinates to the rhodium center, forming a π-complex.Hydrogenation: The coordinated alkene undergoes hydrogenation, resulting in the addition of hydrogen atoms to the double bond and formation of a metal-alkyl intermediate.Reoxidation: The metal-alkyl intermediate reacts with a hydrogen molecule to regenerate the rhodium dihydride species.iv. Starting material, reagent, and solvent:
The starting material is an alkene, and the reagent is Wilkinson's catalyst ([RhCl(PPh3)3]). The reaction is typically carried out in a suitable solvent, such as dichloromethane (CH2Cl2) or tetrahydrofuran (THF).
v. Major and minor end-products:
The major end-product of the hydrogenation reaction is the fully saturated alkane, resulting from the addition of hydrogen across the double bond. The minor end-product may include cis- or trans-configured alkanes if the original alkene substrate possesses geometric isomers.
vi. Intermediates:
The intermediates in the catalytic cycle include:
Rhodium dihydride complex: [RhH2(PPh3)3]Alkene-Rhodium π-complex: [Rh(η2-alkene)(PPh3)3]Metal-alkyl intermediate: [Rh(alkyl)(PPh3)3]These intermediates play a crucial role in facilitating the hydrogenation reaction and enabling the catalytic cycle to proceed.
Learn more about Wilkinson’s catalyst on https://brainly.com/question/31972308
#SPJ1
please need you help aspa

Answers
it took 48 seconds to travel that distance
365m / 48 seconds = 7.61 m/s
the final speed is 7.61 m/s
If 0.100 moles of AgNO₃ react with 0.155 moles of H₂SO₄ according to this UNBALANCED equation below, what is the mass in grams of Ag₂SO₄ that could be formed?
AgNO₃(aq) + H₂SO₄ (aq) → Ag₂SO₄ (s) + HNO₃ (aq)
Answers
Answer:
15.6g Ag2SO4
Explanation:
2AgNO3 + H2SO4 --> Ag2SO4 + 2HNO3
-2x -x
0.1-2x. 0.155-x
x=0.05 x=0.155
0.05mol Ag2SO4 x 311.78g = 15.6g Ag2SO4
Brenda creates an animation that tells the story of a metal from when it is mined as an ore to when it is used as a shiny, protective coating on a car’s iron bumper.
Which two terms will Brenda most likely use in her animation, and in what order?
“electrometallurgy” followed by “electroplating”
“electrometallurgy” followed by “anodization”
“anodization” followed by “electroplating”
“electroplating” followed by “electrometallurgy”
Answers
Answer: A. “electrometallurgy” followed by “electroplating”
Explanation:
Right on edge :)
Answer:
It would be A. “electrometallurgy” followed by “electroplating”
Explanation:
Electrometallurgy is the reduction of metals from compounds to obtain the pure form of metal using electrolysis. In other words, a process in which ores are converted into the pure form of that certain metal through electrolysis.
Electroplating is a process of depositing a thin layer of metal onto an object in an electrolytic cell for strengthening or decorative purposes.
Also in case you are wondering, anodization is a process that makes the surface of metals resistant to corrosion, making the answers containing this process incorrect.
Hope this helps!
I would appreciate brainliest :)
The solubility of gold (V) oxalate, Au2(C2O4)5 is 2.58 g/L. Calculate Ksp from this information.
Answers
Answer:
\(Ksp=3.39x10^{-14}\)
Explanation:
Hello,
In this case, since the dissociation of gold (V) oxalate is:
\(Au_2(C_2O_4)_5(s)\rightleftharpoons 2Au^{5+}(aq)+5(C_2O_4)^{2-}(aq)\)
In such a way, the equilibrium expression is:
\(Ksp=[Au^{5+}]^2[(C_2O_4)^{2-}]^5\)
Thus, since the molar solubility of the gold (V) oxalate is computed by considering its molar mass (834 g/mol):
\([Au_2(C_2O_4)_5]=2.58\frac{g}{L} *\frac{1mol}{834g} =3.09M\)
In such a way, since gold (V) is in a 2:1 molar ratio with the salt and the oxalate in a 5:1 in the chemical reaction, the corresponding concentrations at equilibrium are:
\([Au^{5+}]=3.09x10^{-3}\frac{mol}{L} *\frac{2molAu^{5+}}{1mol} =6.19x10^{-3}M\)
\([(C_2O_4)^{2-}]=3.09x10^{-3}\frac{mol}{L} *\frac{5mol(C_2O_4)^{2-}}{1mol} =0.0155M\)
Therefore, the solubility product turns out:
\(Ksp=(6.19x10^{-3})^2*(0.0155)^5\\\\Ksp=3.39x10^{-14}\)
Regards.
What is a spontaneous change?
A. One that occurs when one specific event happens.
B. One that occurs on its own.
C. One that has a random arrangement of particles.
D. One that has an ordered arrangement of particles.
Answers
Answer:B. One that occurs on its own
Explanation:
Classify the scenarios by the type of error they demonstrate. Systematic error Random error Answer Bank The volume of water in a 50 mL graduated cylinder is recorded by several students in a group. Their recorded volumes are similar, but vary by +0.01 mL. A student weighs a single sample of salt 5 times on the same balance and records 5 measurements that are all different, but within 0.006 g of the initial value. A solid salt compound used for an experiment is contaminated with sugar. A balance consistently reads 0.050 g higher than a set of calibration standards. The percent transmittance of the same solution is measured to be 44.6%, 44.3%, 44.8%, and 44.3%. An incorrectly calibrated instrument gives readings that are consistently low for a set of experiments.
Answers
In scientific measurements, errors can be classified into two main types: systematic errors and random errors. systematic errors can be corrected, random errors can only be reduced by improving the measurement.
Classification of Errors in Scientific MeasurementsIn scientific measurements, errors can be classified into two main types: systematic errors and random errors. Systematic errors are those errors that have a consistent pattern and are introduced by flaws in the measurement process or equipment. An example of a systematic error is an incorrectly calibrated instrument, which gives readings that are consistently low for a set of experiments. Another example is a balance that consistently reads 0.050 g higher than a set of calibration standards. On the other hand, random errors are those that are caused by unpredictable fluctuations in the measurement process and have no consistent pattern. An example of a random error is the measurement of the percent transmittance of the same solution, which was measured to be 44.6%, 44.3%, 44.8%, and 44.3%. A student weighing a single sample of salt 5 times on the same balance and recording 5 measurements that are all different, but within 0.006 g of the initial value, also demonstrates a random error.
To know more about a systematic error and a random error, visit:https://brainly.com/question/14149934
#SPJ4
The chemical reaction that occurs in a gas grill is the combustion of propane, C3H8. Write a balanced equation for this reaction. (Include states of matter.)
Answers
Answer:
\(\Large \boxed{\mathrm{C_3 H_8 \ (g)+5O_2 \ (g) \Rightarrow 3CO_2 \ (g)+4 H_2 O \ (l)}}\)
\(\rule[225]{225}{2}\)
Explanation:
\(\sf C_3 H_8 +O_2 \Rightarrow CO_2 + H_2 O\)
Balancing Carbon atoms on the right side,
\(\sf C_3 H_8 +O_2 \Rightarrow 3CO_2 + H_2 O\)
Balancing Hydrogen atoms on the right side,
\(\sf C_3 H_8 +O_2 \Rightarrow 3CO_2 +4 H_2 O\)
Balancing Oxygen atoms on the left side,
\(\sf C_3 H_8 +5O_2 \Rightarrow 3CO_2 +4 H_2 O\)
\(\rule[225]{225}{2}\)
The combustion reaction of propane is :C₃H₈ \(_(g)\)+5 O₂ \(_(g)\)\(\rightarrow\)3 CO₂\(_(g)\) + 4 H₂O\(_(l)\).
What is combustion reaction?Combustion is defined as a high temperature exothermic reaction between a fuel which acts as a reductant and an oxidant which is usually atmospheric oxygen.It does not result in fire as the flame is visible only when substance which undergoes combustion vaporizes.
Activation energy must be overcome so that combustion is initiated.Solid fuels such as wood and coal initially undergo endothermal pyrolysis which results in gaseous fuels. It is widely used in industry and the application is based on concepts of chemistry, physics and mechanics.
It is a complex chemical process involving many steps which depend on properties of combustible material. It is a type of redox reaction.
Learn more about combustion reactions ,here:
https://brainly.com/question/12172040
#SPJ5
How many moles of aluminum ions al3+ are present in 0.42 mol of al2so43
Answers
There are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
To determine the number of moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3, we need to consider the stoichiometry of the compound.
The formula of aluminum sulfate (Al2(SO4)3) indicates that for every 1 mole of the compound, there are 2 moles of aluminum ions (Al3+). This means that the mole ratio of Al3+ to Al2(SO4)3 is 2:1.
Given that we have 0.42 mol of Al2(SO4)3, we can calculate the moles of Al3+ as follows:
Moles of Al3+ = 0.42 mol Al2(SO4)3 x (2 mol Al3+ / 1 mol Al2(SO4)3)
Moles of Al3+ = 0.42 mol Al2(SO4)3 x 2
Moles of Al3+ = 0.84 mol Al3+
Therefore, there are 0.84 moles of aluminum ions (Al3+) present in 0.42 mol of Al2(SO4)3.
It's important to note that the stoichiometry of the compound determines the mole ratio between the different species involved in the chemical formula. In this case, the 2:1 ratio of Al3+ to Al2(SO4)3 allows us to determine the number of moles of Al3+ based on the given amount of Al2(SO4)3.
For more such question on aluminum visit:
https://brainly.com/question/30451292
#SPJ8
Balance and rewrite the following equation:
C₂H₂ + O2-----> CO₂ + H₂O
Answers
Ethyne gas undergo combustion reaction to give two moles of carbon dioxide and water as per the balanced equation written below:
\(\rm C_{2} H_{2} + \frac{5}{2} O_{2} \rightarrow 2CO_{2} + H_{2}O\)
What is combustion?Combustion is a type of reaction in which a gas burns in oxygen to give water and carbon dioxide. Hydrocarbons alkanes, alkenes or alkynes easily undergo combustion reaction and they can be used as fuels.
C₂H₂ is an alkyne names ethyne and it is an unsaturated hydrocarbon with triple bond between two carbon atoms. Ethyne gas reacts with oxygen to give two moles of carbon dioxide and one mole of water.
To balance the number of carbons the right side carbon dioxide is multiplied by 2 and the number of oxygens is balanced accordingly to get the balanced equation as follows:
\(\rm C_{2} H_{2} + \frac{5}{2} O_{2} \rightarrow 2CO_{2} + H_{2}O\)
To find more on combustion, refer here:
https://brainly.com/question/13153771
#SPJ1
10 Think and answer Scientists discovered layers of sed that was once a seabed. They also discovered several fossils in the layers. Which fossils do you think will be older - those found in the top layers or those found in the bottom layer? Why?
Answers
Sedimentary rocks and volcanic lava are the fossils that will be older, found in the top layers o in the bottom layer.
What is sedimentary rock?The lithified counterparts of sediments are sedimentary rocks. They are primarily created by hardening unconsolidated sediments that already exist via cementing, compacting, as well as other means. Nevertheless, certain types of sedimentary rock do not display an intermediate life as sediment and instead precipitate immediately towards their solid sedimentary form.
Layers or beds are formed by the deposition of volcanic lava flows and sedimentary rocks comprised of mud, sand, gravel, or fossilized shells. The layers just at bottom of the pile typically older than that of the ones at the top because they accumulate over time.
Therefore, sedimentary rocks and volcanic lava are the fossils that will be older, found in the top layers o in the bottom layer.
To learn more about sedimentary rock, here:
https://brainly.com/question/10709497
#SPJ9
will mark brilliant pls help question is above

Answers
Answer:
a.) The garden plants provide energy to all the other organisms. While not every organism consumes garden plants directly, every organism's food lineage can be traced to garden plants. For instance, while ground beetles do not directly consume garden plants, they do eat snails, and snails eat garden plants.
b.) The slug population might increase is the aphid population decreased because there would be less competition for food resources. Both populations consume garden plants, and there is only a finite number of garden plants. Therefore, there is a limit that each population can consume. If there were less aphids eating the garden plants, there would be more left for the slugs. Less starving slugs would lead to greater reproduction and thus a higher slug population.
an automobile gasoline tank holds 40kg of gasoline. when gasoline burns, 140 of oxygen is consumed and carbon dioxide and water are produced. whats the total mass of carbon dioxide and water produced
Answers
40 + 140 = 180 kg of water and carbon dioxide were created in total when gasoline burns.
How much fuel can a tank in a car hold?An average gasoline tank holds 13 to 16 gallons. For instance, the fuel capacity of a Ford Focus is 13.5 gallons, that of an Elantra is 14, and that of a Mazda 3 ranges from 14.9 to 15.9 gallons, depending on the model.
Mass of gasoline plus mass of oxygen equals the combined mass of carbon dioxide and water produced.
combined mass of the water and carbon dioxide produced = 40 + 140 = 180 kg The chemical reaction will appear as follows:
the following equation: fuel + oxygen + carbon dioxide + water + heat
To know more about gasoline burns visit :-
https://brainly.com/question/20514271
#SPJ4
What volume of 0.100 M NaOH is required to precipitate all of the nickel (II) ions from 150.0 mL of a 0.321 M solution of Ni(NO3)2?
Answers
The volume of 0.100 M NaOH is required to precipitate all of the nickel (II) ions from 150.0 mL of a 0.321 M solution of Ni(NO₃)₂ is 483 mL.
What is the volume of the base required?To solve this problem, we need to first write the balanced chemical equation for the reaction between NaOH and Ni(NO₃)₂:
Ni(NO₃)₂ + 2NaOH → Ni(OH)₂ + 2NaNO₃
From this equation, we can see that 2 moles of NaOH are required to precipitate 1 mole of Ni(NO₃)₂.
Therefore, we need to calculate the number of moles of Ni(NO₃)₂ in 150.0 mL of a 0.321 M solution:
moles of Ni(NO₃)₂ = volume (L) x concentration (mol/L)
moles of Ni(NO₃)₂ = 0.150 L x 0.321 mol/L
moles of Ni(NO₃)₂ = 0.0483 mol
To precipitate all of the nickel (II) ions, we need to add an equal number of moles of NaOH.
Since the concentration of NaOH is 0.100 M, we can calculate the volume of NaOH required:
moles of NaOH = 0.0483 mol (from above)
volume of NaOH = moles of NaOH / concentration of NaOH
volume of NaOH = 0.0483 mol / 0.100 mol/L
volume of NaOH = 0.483 L or 483 mL
Learn more about volume of base here: https://brainly.com/question/21589292
#SPJ1
Students want to gather evidence for the claim that the number of atoms present before a chemical reaction is equal to the number of atoms present after the chemical reaction. They decide to react vinegar and baking soda in a sealed plastic bag. Which of the following will provide the evidence the students need?
A. The mass of the plastic bag, baking soda, and vinegar before the reaction was equal to the mass after the reaction.
B. Bubbles were produced during the reaction, which meant that a gas was being produced.
C. The plastic bag did not change in any way, indicating that it was not involved in the reaction.
D. The mass of the baking soda was exactly equal to the mass of the vinegar used to create the chemical reaction. THIS IS DUE IN AN HOUR HELPPP!!!
Answers
The mass of the plastic bag, baking soda, and vinegar before the reaction was equal to the mass after the reaction. Option A.
Law of conservation of atomsAtoms, mass, and energy are conserved during chemical reactions. Even though their forms may change after reactions.
For a reaction involving vinegar and baking soda, the equation can be expressed as:
vinegar + baking soda ---> carbon dioxide + sodium acetate + water
or
\(CH_3COOH (aq) + NaHCO_3 (aq) --- > CH_3COONa (aq) + H_2O (l) + CO_2 (g)\)
In other words, sodium acetate, water, and carbon dioxide gas are produced in the reaction. Assuming that the reaction vessel is not sealed, the gas would escape away from the vessel.
Since the vessel was sealed, the mass of the plastic bag, baking soda, and vinegar before the reaction will be equal to the sum of the masses of the plastic bag and the products after the reaction.
This will show that the number of atoms present before the reaction is the same as the number of atoms present after the reaction.
More on the law of conservation of atoms can be found here: https://brainly.com/question/20635180
#SPJ1
PLEASE HELP QUICKK
Calculate the energy of combustion for one mole of butane if burning a 0.367 g sample of butane (C4H10) has increased the temperature of a bomb calorimeter by 7.73 °C. The heat capacity of the bomb calorimeter is 2.36 kJ/ °C.
Answers
The energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
To calculate the energy of combustion for one mole of butane (C4H10), we need to use the information provided and apply the principle of calorimetry.
First, we need to convert the mass of the butane sample from grams to moles. The molar mass of butane (C4H10) can be calculated as follows:
C: 12.01 g/mol
H: 1.01 g/mol
Molar mass of C4H10 = (12.01 * 4) + (1.01 * 10) = 58.12 g/mol
Next, we calculate the moles of butane in the sample:
moles of butane = mass of butane sample / molar mass of butane
moles of butane = 0.367 g / 58.12 g/mol ≈ 0.00631 mol
Now, we can calculate the heat released by the combustion of the butane sample using the equation:
q = C * ΔT
where q is the heat released, C is the heat capacity of the calorimeter, and ΔT is the change in temperature.
Given that the heat capacity of the bomb calorimeter is 2.36 kJ/°C and the change in temperature is 7.73 °C, we can substitute these values into the equation:
q = (2.36 kJ/°C) * 7.73 °C = 18.2078 kJ
Since the heat released by the combustion of the butane sample is equal to the heat absorbed by the calorimeter, we can equate this value to the energy of combustion for one mole of butane.
Energy of combustion for one mole of butane = q / moles of butane
Energy of combustion for one mole of butane = 18.2078 kJ / 0.00631 mol ≈ 2888.81 kJ/mol
Therefore, the energy of combustion for one mole of butane is approximately 2888.81 kJ/mol.
In conclusion, by applying the principles of calorimetry and using the given data, we have calculated the energy of combustion for one mole of butane to be approximately 2888.81 kJ/mol.
For more questions on molar mass, click on:
https://brainly.com/question/837939
#SPJ8
How many moles of
Cts are needed to make
15.5 moles of CO₂? How
much O2 will be needed?
0₂
C₂Hs +50₂3CO₂ + 4H₂O
Answers
1. The number of mole of C₃H₈ needed to make 15.5 moles of CO₂ is 5.2 moles
2. The number of moles of O₂ needed is 25.8 moles
1. How do I determine the number of mole of C₃H₈ needed?The number of mole of C₃H₈ needed can be obtained as illustrasted below:
C₃H₈ + 5O₂ -> 3CO₂ + 4H₂O
From the balanced equation above,
3 moles of CO₂ was obtained from 1 mole of C₃H₈
Therefore,
15.5 moles of CO₂ will be obtain from = (15.5 × 1) / 3 = 5.2 moles of C₃H₈
Thus, number of mole of C₃H₈ needed is 5.2 moles
2. How do I determine the number of mole of O₂ needed?We can obtain the number of mole of O₂ needed as follow:
C₃H₈ + 5O₂ -> 3CO₂ + 4H₂O
From the balanced equation above,
3 moles of CO₂ was obtained from 5 moles of O₂
Therefore,
15.5 moles of CO₂ will be obtain from = (15.5 × 5) / 3 = 25.8 moles of O₂
Thus, number of mole of O₂ needed is 25.8 moles
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
Complete question:
How many moles of C₃H₈ are needed to make
15.5 moles of CO₂? How much O₂ will be needed?
C₃H₈ + 5O₂ -> 3CO₂ + 4H₂O
How many moles in 5 grams?
Answers
Answer:
The molar mass of atoms of an element is given by the standard relative atomic mass of the element multiplied by the molar mass constant
Explanation:
You are out hiking on a cold snowy day. You put on your battery-heated socks. In which direction is the thermal energy flowing?
There is no thermal energy in this scenario.
Thermal energy is moving from the air to your socks
Thermal energy is moving from your feet to your socks
Thermal energy is moving from your socks to your feet
Answers
The correct answer is that thermal energy is moving from your feet to your socks. The battery-heated socks work by using the heat generated by your body to warm your feet in cold weather.
The body produces heat, which is converted into thermal energy, and moves from your feet to the socks.
In order to warm your feet and make them more comfortable in cold weather, the socks use thermal energy. The thermal energy is only transferred in one direction, from your feet to the socks.
No more energy is produced because the battery-heated socks utilise your body's thermal energy to keep your feet warm.
To learn more about thermal energy visit:
https://brainly.com/question/19666326
#SPJ1
1. Which substance is reduced in this redox reaction?
CuO(s) + H₂(g) → Cu(s) + H₂O(l)
A. H₂
B. Cu
C. H₂0
D. o
Answers
Answer:
B.Cu
Explanation:
n the reaction H2 is oxdized since it has lost electrons and Cu is reduced since it has gained electrons
What is a solution?
OA. The substance that dissolves another substance
OB. Two liquids that do not mix with each other
OC. The substance that is dissolved in another substance
OD. The mixture of one substance dissolved in another
Answers
Answer: the substance in which another substance
Explanation:
done