How would you describe the way the human blood cells look in the low power setting versus the high power setting?
Answers
Human blood appears to be a red liquid to the naked eye, but under a microscope we can see that it contains four distinct elements:
plasma red blood cells white blood cells and plateletsIamSugarBee
Related Questions
a scientist conducts an experiment to determine the rate of the following reaction: if the initial concentration of n2 was 0.400 m and the concentration of n2 was 0.350 m after 0.100 s, what is the average rate of reaction over the first 100 milliseconds?
Answers
After 0.100 s, the average rate of reaction over the first 100 milliseconds is 0.25 mol s^-1. if the initial concentration of n2 was 0.400 m and the concentration of n2 was 0.350 m.
The average rate of reaction over the first 100 milliseconds when the initial concentration of N2 was 0.400 M and the concentration of N2 was 0.350 M after 0.100 s can be calculated as follows:
Average rate of reaction = {N2 consumed or produced in mol} / {time in seconds}
The balanced chemical equation for the reaction is:
N2(g) + 3H2(g) → 2NH3(g)
As per the given equation, one mole of N2 reacts to produce two moles of NH3. So, the mole of N2 consumed in the reaction would be equal to half the mole of NH3 produced.
Therefore, mole of N2 consumed = (1/2) × (0.050 M) = 0.025 M
Now, the average rate of reaction can be calculated as follows:
Average rate of reaction = {N2 consumed or produced in mol} / {time in seconds}
= 0.025 mol / 0.100 s
= 0.25 mol s^-1
Therefore, the average rate of reaction over the first 100 milliseconds is 0.25 mol s^-1.
For more such questions on rate of reaction , Visit:
https://brainly.com/question/24795637
#SPJ11
Thorium-234 undergoes beta decay to form a daughter nuclide and a beta particle. What are the mass number and atomic number for the daughter nuclide?
A.230, 88
B.234, 89
C.230, 91
D. 234, 91
Answers
The mass number of the daughter nuclide will remain the same as Thorium-234 (mass number 234). The correct answer is D. 234, 91.
When there are too many protons or neutrons in a nucleus, one of the protons or neutrons will turn into the other, which is known as beta decay. During beta minus decay, a neutron transforms into a proton, electron, and antineutrino.
A daughter nuclide is created when a neutron in the nucleus decays into a proton during beta decay. While the mass number stays constant, the atomic number rises by 1.
We know the daughter nuclide will have an atomic number one unit higher than thorium (atomic number 90) because thorium-234 (Th-234) undergoes beta decay. The daughter nuclide will continue to have the same mass number as thorium-234 (mass number 234).
To know more about beta decay:
https://brainly.com/question/4184205
#SPJ4
PLEASE ANSWER QUICK RIGHT ANSWERS ONLY WILL MARK BRAINLIEST

Answers
Explanation:
To find the freezing point of the solution, we can use the freezing point depression equation:
ΔT = Kᵣ x m
Where ΔT is the change in freezing point, Kᵣ is the freezing point depression constant of benzene, and m is the molality of the solution.
Substituting the values from the problem, we get:
ΔT = 5.12 °C/m x 2.8 m
ΔT = 14.34 °C
Since ΔT = Tᵢ - T, where Tᵢ is the freezing point of the solvent (benzene) and T is the freezing point of the solution, we can rearrange the equation to solve for T:
T = Tᵢ - ΔT
T = 5.50 °C - 14.34 °C
T = -8.84 °C
Therefore, the freezing point of the solution is -8.84 °C.
Answer:The freezing point of the solution is -8.84 °C.
Explanation:
a carbon-fe alloy containing 1.5 wt% c is cooled down to 800oc. determine what phases are present, the fraction of each phase present, and the composition of each phase. p25
Answers
To determine the phases present, a fraction of each phase, and the composition of each phase in a carbon-fe alloy containing 1.5 wt% C cooled down to 800°C, you would need to refer to the phase diagram for carbon-iron (Fe-C) alloy, also known as the iron-carbon phase diagram.
1. Consult the phase diagram: Look for the region that corresponds to the composition of the alloy, which is 1.5 wt% C.
Find the temperature range of 800°C.
2. Determine the phases present: From the phase diagram, identify the phases present at 800°C for an alloy with 1.5 wt% C.
3. Determine the fraction of each phase present: The phase diagram may provide information about the fraction of each phase present at 800°C for the given composition.
4. Determine the composition of each phase: The phase diagram should also indicate the composition of each phase present at 800°C.
Please refer to the specific phase diagram for the carbon-fe alloy you are working with to find the exact information on phases, fractions, and compositions at 800°C for an alloy with 1.5 wt% C.
Read more about Phase diagram.
https://brainly.com/question/31251128
#SPJ11
Zinc chloride + Magnesium →
Answers
Answer:
im assuming you need the balanced equation
Explanation:
Zn + MgCl2 = ZnCl2 + Mg
Answer:
it will mgcl2 + zn
Explanation:
Mg will replace zn from its salt solution
to form mgcl2
hope it helps
What is the Molar Mass of BaCl2?
Answers
constant temperature and the pressure upon it. Write down the relationship in terms of a (a) Proportion (b) equation (c) formula
Answers
Answer:
Proportion : The Equality of two ratios
Equation: Values of two mathematical expressions are equal.
Formula: Mathematical relationship or rule expressed in symbols.
Explanation:
A sample of ground beef contains 21.3% protein and 16.9% fat, both by mass. What mass of this ground beef, in grams, contains 350 Calories? Assume all Calories come from protein and fat.
Answers
Answer:
147.5 grams
Explanation:
Let the required mass of ground beef be m .
protein in it = .213 m
fat in it = .169 m
1 gram of protein gives 4 cals .
1 gram of fat gives 9 cals .
cals given by .213 m protein = 4 x .213 m .
cals given by .169 m fat = 9 x .169 m .
4 x .213 m + 9 x .169 m = 350
.852 m + 1.521 m = 350
2.373 m = 350
m = 147.5 grams Ans
What is the mass number of sodium

Answers
Answer:
Explanation:
Sodium mass number 23, 11 electrons
Magnesium: neutrons = 12
aluminum : atomic number = 13
phosporus : protons = 15
Answer:
23
Explanation:
You need to add protons and neutrons to get mass
120g of C₂H, react with 288g of O₂, What is the limiting reactant? How many grams of water can be produced? How many grams of excess? If 130 grams of water are actually produced, what is the percent yield?
Answers
The percentage yield is equal to 200.6%.
The balanced equation for the given chemical reaction is :
2C2H + 5O2 → 4CO2 + 2H2O
Let's find the limiting reagent:
Mass of C2H = 120 g
Mass of O2 = 288 g
Molar mass of C2H = 26 g/mol
Molar mass of O2 = 32 g/mol
The number of moles of C2H is equal to:
120 g × 1 mol/26 g = 4.62 mol
The number of moles of O2 is equal to:
288 g × 1 mol/32 g = 9 mol
According to the stoichiometry of the balanced equation, 2 moles of C2H react with 5 moles of O2, so:
If 4.62 moles of C2H is used, then the required amount of O2 would be:
5/2 × 4.62 mol = 11.6 mol
We only have 9 mol of O2 which means it is the limiting reagent.
C2H is the excess reagent.
Let's find the mass of water produced:
According to the stoichiometry of the balanced equation,2 moles of H2O is produced per 2 moles of C2H2 moles of H2O is produced per 5 moles of O2If 9 mol of O2 is used, the number of moles of water produced would be:
2/5 × 9 mol = 3.6 mol
The mass of water produced is equal to:
3.6 mol × 18 g/mol = 64.8 g
Therefore, 64.8 g of water can be produced.
The amount of excess reagent is equal to:
4.62 mol - 2/2 × 9 mol = 4.62 - 9
= -4.38 mol
C2H is the excess reagent and it is not possible to have negative amount of a substance.
So, we assume that there is no excess reagent.
If 130 grams of water is produced, then the percentage yield is equal to:
Percent yield = actual yield/theoretical yield × 100
Theoretical yield is equal to 64.8 g
Actual yield is equal to 130 g
Percent yield = 130/64.8 × 100
= 200.6%
Therefore, the percentage yield is equal to 200.6%.
For such more questions on percentage yield
https://brainly.com/question/11963853
#SPJ8
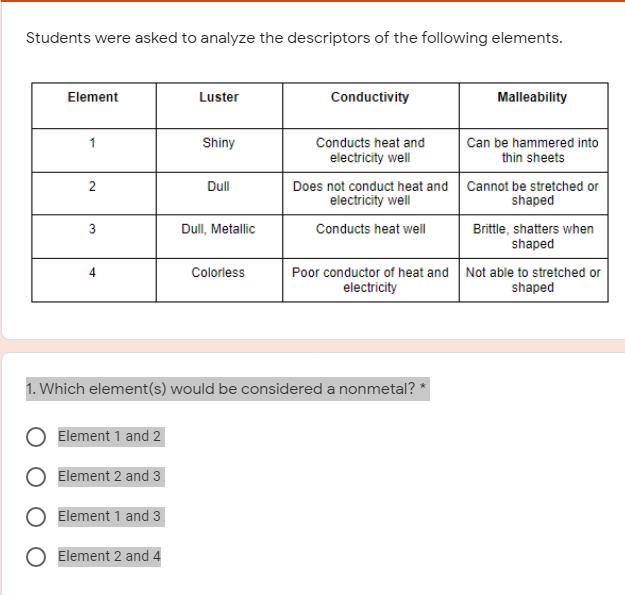
Metals, Non-Metals, Metalloids
Students were asked to analyze the descriptors of the following elements.

Answers
what is single and double displacement reaction?
Answers
\({{\boxed{\mathcal{\red{It \: will \: be \: in \: your\: \: text \: book \: see}}}}}\)
Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
In general, what happens when a subscript is found
outside of parentheses?
Answers
Answer:
you multiply it by the other subscripts in the parenthesis.
Explanation:
help plz number 11 please no links if don't know plz don't answer

Answers
Same thing, but upside down

Answer:
Drain cleaner pH =12.10 Basic
Hand soap pH = 9.4 Basic
Blood pH =7.35 Basic
spit pH =6.5 acidic
water pH 7.0 neutral
milk pH 6.6 acidic
chicken soup 6.8 acidic
cofee ph 4.9 acidic
orange juice pH 3.5 acidic
soda pop ph 2.5 acidic
vomit 1.2 acidic
battery acide 1.0 highly acidic
What MASS of NaCl are required to make 2.69L of a 0.14M solution?Use the correct abbreviation for the UNITS
Answers
To solve this problem, let's use the definition for molarity:
Replacing the values of the problem:
Now, to find the mass, we multiply by the molecular weight of NaCl. (Which is about 58.44g/mol)
The answer is approximately 22.2g of NaCl



A 1000 liter tank initially contains 400 liters of a solution saline A in which 20 kg of salt are dissolved. Determine the concentration rho of another salt solution B, such that, if solution B is poured at a rate of 5 l/min, and the well-mixed solution leaves the tank at At a rate of 3 l/min, the solution in the tank reaches a concentration of 0.12 kg/l after 1 hour.
Answers
150, tank, concentration
A tank filled with 1000 liters contains 400 liters of a solution saline A in which 20 kg of salt are dissolved then,
The solution for the concentration rho is 0.12 kg/l.
Therefore, the rate of salt being added to the tank is equal to the rate of salt being removed from the tank.
Using the above formula, the concentration of solution B can be calculated.
Let rho be the concentration of solution B.Concentration of saline A is given bymass of salt/volume of solution = 20/400 = 0.05 kg/l
Concentration of saline A after t minutes of mixing with saline B of concentration rho = (20 + 0.1 * rho * t)/(400 + (t * (5 - 3)))In 1 hour = 60 minutes, concentration = 0.12 kg/l
Therefore, we can say:(20 + 0.1 * rho * 60)/(400 + (60 * (5 - 3))) = 0.12
By solving this equation, we get rho = 150 kg/m3.
Answer: 150, tank, concentration
learn more about tank filled on
https://brainly.com/question/16855905
#SPJ11
Rearrange the elements in the order of increasing first ionization energy. Place the element symbol with the lowest first ionization energy at the top, and the element with the highest first ionization energy at the bottom. I Br F CI
Answers
Answer:
I,Br,Cl,F
Explanation:
ionization energy decreases the farther down an element is
Consider the reaction, CS2(l)+3O2(g)→CO2(g)+2SO2(g) . The rate of change of CS2(g) is – 0.012 M/s . What is the rate of change of SO2(g) ?
Answers
The rate of change of \(SO_2\)(g)=0.024M/s when the rate of change of \(CS_2(g)\) is – 0.012 M/s.
What is the rate of reaction?Reaction rate, in chemistry, is the speed at which a chemical reaction proceeds.
\(CS_2(l)+3O_2(g)\) → \(CO_2(g)+2SO_2(g)\)
\(Rate = \frac{-dcs_2}{dt} =\frac{-do_2}{dt} =\frac{+dco_2}{dt} =\frac{1dso_2}{dt}\)
Rate = \frac{-dcs_2}{dt} =\frac{-do_2}{dt} =\frac{+dco_2}{dt} =\frac{1dso_2}{dt}
Given:
\(\frac{-dcs_2}{dt} =-0.012 M/s\)
\(\frac{-dcs_2}{dt} =\frac{dso_2}{dt}\)
\(\frac{dso_2}{dt}=0.024M/s\)
The rate of change of \(SO_2\)(g)=0.024M/s
Hence, the rate of change of \(SO_2\)(g)=0.024M/s
Learn more about the rate of change here:
https://brainly.com/question/13103052
#SPJ1
H2(g) + I2(g) ↔2HI(g) + heat. If more I2 is added, in what direction will the equilibrium shift? Group of answer choices
Answers
Answer:
Towards the products, or to the right
Explanation:
There are no provided answer choices, but the answer should be to the right.
By Le Chatelier's principle, which basically can be summarized as "if you mess with chemistry, chemistry messes back", if more reactants are added, the equilibrium will shift to the right towards the products in order to make more products and counteract the increase in I₂.
What is the specific heat of a substance if a mass of 10. 0 kg increases in temperature from 10. 0°C to 70. 0°C when 2,520 J of heat is applied? Use q equals m C subscript p Delta T. 0. 00420 J/(gi°C) 0. 00661 J/(gi°C) 238 J/(gi°C) 252 J/(gi°C).
Answers
To determine the specific heat of a substance using the given information -
the formula q = m * C * ΔT, where q represents the heat energy transferred, m is the mass of the substance, C is the specific heat, and ΔT is the change in temperature, we can rearrange the formula to solve for C: C = q / (m * ΔT) Plugging in the values: q = 2520 J m = 10.0 k ΔT = (70.0°C - 10.0°C) = 60.0°C C = 2520 J / (10.0 kg * 60.0°C) Calculating the value C = 2520 J / (600.0 kg·°C) C ≈ 4.20 J/(g·°C) Therefore, the specific heat of the substance is approximately 4.20 J/(g·°C).
learn more about:- specific heat of a substance here
https://brainly.com/question/21184946
#SPJ11
A balloon with no molecule inide of it i placed on top of a flak that wa filled with ethanol vapor at a temperature of 270 K. It wa determined that the flak ha a total volume of 150 mL. What would be the volume of the balloon after the flak wa heated to a temperature of 330 K? The quetion i aking for the volume of the balloon after heating, not the total volume of the flak and the balloon
Answers
The volume of the balloon after heating, not the total volume of the flak and the balloon is 0.18 L.
An ideal gas is a theoretical gas composed of many randomly transferring factor particles that aren't difficult to interparticle interactions. the best gasoline idea is beneficial because it obeys the precise gas law, a simplified equation of country, and is amenable to evaluation under statistical mechanics.
Volume is a degree of occupied three-dimensional space. it's far more frequently quantified numerically the usage of SI-derived gadgets or by way of diverse imperial gadgets. The definition of length is interrelated with the extent.
Using the ideal gas equation:-
Given;
V₁ = 150 mL = 0.150 L
T₁ = 270 K
V₂ = ?
T₂ = 330 K
V₁/T₁ = V₂/T₂
V₂ = V₁T₂/T₁
= 0.150 × 330 / 270
= 0.18 L
Learn more about ideal gas here:-https://brainly.com/question/20348074
#SPJ4
How many cubic inches are there in 426 mL
Answers
Carbon tetrachloride (CCIA) is: covalent or Ionic?
Answers
Answer:
covalent
Explanation:
What’s the ph of the following equation

Answers
Answer: The pH of [H30+] = 2.4 * 10 -3 M is 2.6
Explanation:
pH = 3 - log 2.4 = 2.6
11.
What is the missing product from this reaction?
32 32
15P → 16S +
a.
4.
2 He
b.
0
10
C.
0
or
d.
0
le
e.
0
1P

Answers
Answer:
good times
Explanation:
always nedded
Here the nuclear decay of phosphorous to give S-32 is an example of beta-decay. Hence, the emitted particle is an electron. Thus, option b is correct.
What is beta decay ?The heavy unstable radioactive isotopes undergo nuclear decay by the emission of alpha or beta particle. Thus, there are mostly two types of decays, alpha decay and beta decay.
In alpha decay, the isotope emits a helium nuclei. Hence, mass number decreases by 4 units and atomic number decreases by 2 units. In beta decay, mass number does not change but atomic number increases by 1.
Here, P-32 undergo beta -decay by emitting an electron to form S -32. Hence, atomic number is increased by one. Therefore, the mission product is an electron option b.
Find more on beta- decay:
https://brainly.com/question/12448836
#SPJ6
Which formula shows a correct representation of the combined gas law?(1 point)
A. V1 / P1T1=V2 / P2T2
B. P1 / V1T1=P2 / V2T2
C. V1T1 / P1=V2T2 / P2
D. V1P1 / T1=V2P2 / T2
Answers
The formula that shows the correct representation of the combined gas law is; V1P1 / T1=V2P2 / T2
The combined gas law is obtained from the statements of Charles law and Boyle's law as follows;
Charles law; V/T = k
Boyle's law = PV = k
Combining the two I have; PV/T = k
For two masses of an ideal gas;
V1P1 / T1=V2P2 / T2
This is the statement of the combined gas law.
Learn more: https://brainly.com/question/1190311
Gases Unit Test Honors Chemistry A
1) B. The volume increases to twice its original value.
2) A. volume and temperature directly proportional
pressure and volume inversely proportional
pressure and temperature directly proportional
3) D. Gay-Lussac’s law, by seeing how changes in temperature affect the pressure of the gas
4) B. V1P1/T1=V2P2/T2
5) A. It is a straight line with a positive slope showing that an increase in temperature results in an increase in volume.
6) D. keeping the pressure constant and increasing the temperature
7) A. When temperature is held constant and volume increases, the pressure increases.
8) D. pressure, volume, temperature, number of moles
9) A. volume
10) A. 7.10 L/mol
11) A. 0.105 mol
12) B. 27 g/mol
13) B. the temperature increasing by a factor of 2
14) brainly.com/question/24915187
15) brainly.com/question/24544023
16)brainly.com/question/24544061
17) brainly.com/question/24598785
Geologists obtain indirect evidence about Earth’s interior by
a.
measuring pressure differences at Earth’s surface.
b.
estimating temperature inside earth.
c.
directly looking under the many layers.
d.
recording and studying seismic waves.
Answers
Answer:
I'm sure it's D.
Explanation:
They obtain evidences by recording and studying seismic waves.
what chemical is mixed with water to clean the scba
Answers
The chemical commonly mixed with water to clean Self-Contained Breathing Apparatus (SCBA) equipment is typically a disinfectant or cleaning solution.
There are various cleaning solutions available specifically designed for cleaning SCBA equipment. The specific chemical used may vary depending on the manufacturer or department protocols. Some common examples of cleaning agents used for SCBA cleaning include:
1. Quaternary Ammonium Compounds (Quats): Quats are a type of disinfectant that can effectively kill bacteria, viruses, and fungi. They are often used as cleaning agents for SCBA equipment due to their antimicrobial properties.
2. Hydrogen Peroxide: Hydrogen peroxide is a commonly used disinfectant and cleaning agent. It has strong oxidizing properties that can help kill bacteria and viruses on SCBA surfaces.
3. Isopropyl Alcohol: Isopropyl alcohol, also known as rubbing alcohol, is often used as a cleaning agent for SCBA equipment. It has antimicrobial properties and can help remove dirt, grime, and bacteria from surfaces.
4. Detergent Solutions: Mild detergent solutions may also be used for cleaning SCBA equipment. These solutions help remove dirt, oils, and contaminants from the surface of the equipment.
To know more about cleaning solution refer here:
https://brainly.com/question/31755818?#
#SPJ11
What is that Molar mass of ammonium phosphite?
Answers
Answer:
149.09 g/mol GOOD LESSONS ♡
Answer:
The answer is 149.09 g/mol
Explanation:
I hope this helps!!