i will mark the brainiest answer
Radioactive CsCl is used in nuclear medicine as a tracer. What would you call CsCl?
cerium chloride
cesium chloride
cesium chlorine
cesium monochloride
MgS has been found in meteorites. What would you call MgS?
a.rubidium phosphide
b.rubidium phosphate
c.rubidium phosphite
d.triubidium monophosphate
Answers
Related Questions
Lily replicates an experiment that found that the number of calories in a particular food is 50 kcal. She obtained data from
five trials: 50 kcal 72 kcal, 50 kcal, 12 kcal, and 50 kcal. Which best desribes her data results? A. accurate B. incorrect C. invalid D. precise
Answers
Answer:
invalid
Explanation:
Just imagine doing this experiment MULTIPLE TIMES and one of the trials you get 72 Kcal while in another u get 12kcal. It doesn't make sense. Somewhere in the experiment she went wrong. So its invalid
Kim loại đòng sắt đc tạo từ nguyên tố nào
Answers
Answer:
sup brö how is it back home?
Explanation:
just curious you know
Objects with potential energy got that energy:
A. Entirely from their height above the earth.
B. After work was performed on them.
C. When kinetic energy was released from fossil fuels.
D. Through electromagnetic discharges.
Answers
Answer:
D. Through electromagnetic discharges.
Explanation:
sodium bisulfite converts bromine (br2) to bromide (br-). sodium bisulfite is a(n)
Answers
Sodium Bisulfite converts Bromine (br2) to Bromide (br-). Sodium Bisulfite is a reducing agent.
In chemistry, a reducing agent is a chemical species that "donates" an electron to an electron acceptor. Examples of substances that are normally reducing agents include earth metals, formic acid, oxalic acid, and sulfite compounds. Reducing and oxidizing agents are responsible for corrosion, or "decomposition of metals by electrochemical activity." Corrosion requires an anode and a cathode.
Strong reducing agents are electropositive elements that can readily donate electrons in chemical reactions. Sodium, hydrogen and lithium are examples of strong oxidants. Weak reducing agents react less violently than strong reducing agents, but can participate in reactions that produce heat and gaseous products that pressurize the closed vessel and can participate in further reactions.
To learn more about reducing agents, here
https://brainly.com/question/2890416
#SPJ4
Which of the following is NOT a part of adenosine diphosphate?
glucose, ribose, adenine, two phosphate groups
Answers
Answer:
a. glucose c. ribose b. adenine d. two phosphate groups user: all organisms need energy to perform different functions. cells are able to ...
students dissolved a compound in water and added hydrochloric acid. a precipitate was formed. the precipitate dissolved in boiling water. what was the precipitate?
Answers
It is difficult to determine the identity of the precipitate without more information about the compound that was dissolved in water.
However, based on the fact that the precipitate dissolved in boiling water, it is likely that the precipitate was a salt or an ionic compound that is more soluble in hot water than in cold water.
To know more about precipitate refer here:
https://brainly.com/question/18109776
#SPJ11
can the following compounds undergo sn1 reactions? why or why not? support your answers by drawing the corresponding electronic effects in each structure.
Answers
Whether a compound can undergo SN1 reactions depends on its molecular structure and electronic effects.
SN1 reactions involve a two-step mechanism where the leaving group dissociates first, creating a carbocation intermediate. Compounds that can stabilize carbocations through resonance or hyperconjugation are more likely to undergo SN1 reactions. In this case, we need to analyze the structures of the given compounds and evaluate their ability to form stable carbocation intermediates.
To determine if a compound can undergo SN1 reactions, we need to consider its ability to stabilize carbocation intermediates. Factors such as resonance, hyperconjugation, and neighboring electron-withdrawing or electron-donating groups play a crucial role in stabilizing carbocations.
By examining the structures of the given compounds and their electronic effects, we can determine their potential for SN1 reactions. Drawing the corresponding electronic effects for each structure allows us to analyze the presence or absence of stabilizing factors.
For compounds with resonance structures that involve delocalization of the positive charge, such as allylic or benzylic carbocations, they are more likely to undergo SN1 reactions due to the increased stability provided by resonance.
On the other hand, compounds lacking resonance or neighboring electron-donating groups might not undergo SN1 reactions easily, as they lack stabilizing factors for carbocation formation. These compounds may instead favor SN2 reactions, where the nucleophile directly attacks the substrate, bypassing the formation of a carbocation intermediate.
By considering the molecular structure and the presence or absence of stabilizing factors, we can determine if a compound is likely to undergo SN1 reactions or not.
Learn more about structure here;
brainly.com/question/33100618
#SPJ11
Can the following compounds undergo SN1 reactions? Why or why not? Support your answers by drawing the corresponding electronic effects in each structure. Br Br or
What is the most abundant greenhouse gas released through human activities? a. methane b. nitrous oxide c. carbon dioxide d. sulfur dioxide
Answers
Answer:
C. Carbon dioxide. (C02.)
Explanation:
Hope this helps. :)
Answer:
Carbon dioxide
Explanation:
an amount of medication of mg is found to result in a blood pressure of mm hg. what is the predicted blood pressure
Answers
The predicted blood pressure when an amount of medication of 186mg is found to result in a blood pressure of 125.35 mm Hg would be 127.977 mm Hg.
What is regression line?The regression line is a straight line that is used to explain how a dependent variable (y) changes in response to the change in an independent variable (x) with the help of the slope and y-intercept. In other words, a regression line is an equation for a line of best fit for the given set of data.
The regression line equation is as follows: Y^ = a + bx Here, "a" represents the y-intercept, and "b" represents the slope of the regression line. We have given the equation of the regression line as follows: Y^ = 140 + (-0.0667)X. Now, we have been asked to find the predicted blood pressure when an amount of medication of 186mg is found to result in a blood pressure of 125.35 mm Hg.
To find out the predicted blood pressure, we have to substitute the value of "X" in the regression line equation. Y^ = 140 + (-0.0667)X Y^ = 140 + (-0.0667)186 = 127.977.
Therefore, the predicted blood pressure when an amount of medication of 186mg is found to result in a blood pressure of 125.35 mm Hg would be 127.977 mm Hg.
To know more about blood pressure click on below link:
https://brainly.com/question/4215574#
#SPJ11
complete question :
A medical researcher wants to determine how a new medication affects blood pressure.The equation of the regression line is Y^=140+(-0.0667)X
An amount of medication of 186mg is found to result in a blood pressure of 125.35 mm Hg. What is the predicted blood pressure_____mm Hg.
Which process describes the wearing
away of rock?
A. drainage
B. erosion
C. infiltration
D. weathering
Answers
Answer:
weathering
Explanation:
hope this helps
Complete and balance the following redox reaction in basic solution. Be sure to include the proper phases for all species within the reaction. CIO (aq) + CO2(aq) → CIO₂(g) + CO₂(g)
Answers
The balanced redox reaction in basic solution is: \(CIO(aq) + CO_2(aq) + OH^{-(aq)} \rightarrow CIO_2(g) + CO_2(g) + H_2O(l)\)
To balance this redox reaction in basic solution, we first need to identify the oxidation state of each element in the equation. We see that the oxidation state of chlorine changes from +1 to +4, while the oxidation state of carbon changes from +4 to +2.
Next, we balance the equation in acidic solution, as we normally would, and then add OH⁻ to both sides of the equation to neutralize the H⁺ ions and form water molecules. This adds an equal number of H⁺ and OH⁻ ions to both sides of the equation, so the charge balance is maintained.
After balancing the equation in basic solution, we make sure that the number of atoms of each element is the same on both sides, and that the charges are balanced. Finally, we add the phases of each species to complete the equation.
To know more about redox reaction, refer here:
https://brainly.com/question/13293425#
#SPJ11
3. Hydrogen peroxide forms gas bubbles when it is added to blood.
The other reaction product is water. Inserting a glowing splint
into a sample of this gas causes the splint to relight. 20
(a) Identify the gas.
(b) Classify the reaction.
(c) Write a balanced chemical equation for this reaction.
Answers
The gas is oxygen which causes the splint to relight and the reaction is of hydrogen peroxide resulting into water and oxygen.
Why hydrogen peroxide forms gas bubbles when it is added to blood?Hydrogen peroxide on reaction and decomposition releases water and oxygen .The reaction followed is something like this: 2H2O2= 2H2O + O2 , putting hydrogen peroxide on a cut.And the bubbles are actually symptomizing that the solution is killing bacteria that is it prevents bacteria to grow.And that solution is true solution which consist of various components in the reaction that is being done.Hence the gas is oxygen , and the reaction is 2H2O2= 2H2O + O2, and its the oxygen gas causing the splint to relight.To know more about hydrogen peroxide visit:
https://brainly.com/question/18709693
#SPJ13
Help!!! Answer
Imagine your teacher asks you to design an experiment where you test the effect of temperature on the growth of a plant. You have 5 plants that you plant and place in different temperatures around the room. What would your one variable be and what would your constants be?
Answers
Dependent variables could be height, number of leaves, biomass, etc. The constants could be the amount of water fed to the plants and other environmental conditions apart from the temperature.
Experimental variablesExperimental variables could be independent, dependent, or constant.
Independent variables are supplied by the researcher and are often varied or manipulated to produce different effects on experimental systems or subjects.
Dependent variables are measured. Their values are often affected by whatever independent variable the researcher supplies.
Constant variables are uniform throughout the experimental groups or subjects.
Thus, in this case, the aim is to test the effect of temperature on the growth of a plant. The independent variable is the different temperatures.
The dependent variable would be any feature of the experimental plants that indicate growth. It could the height, the number of leaves, etc.
The constant variable would be other conditions the experimental plants are subjected to.
More on experimental variables can be found here: https://brainly.com/question/6350073
#SPJ1
When 40. 0 mL of 1. 00 M H2SO4 is added to 80. 0 mL of 1. 00 M NaOH at 20. 00°C in a coffee cup calorimeter, the temperature of the aqueous solution increases to 29. 20°C. If the mass of the solution is 120. 0 g and the specific heat of the calorimeter and solution is 4. 184 J/g • °C, how much heat is given off in the reaction? (Ignore the mass of the calorimeter in the calculation. ) Use q equals m C subscript p Delta T. 4. 62 kJ 10. 0 kJ 14. 7 kJ 38. 5 kJ.
Answers
The heat given off in the reaction is 4.62 kJ. This corresponds to the first option in the list of choices.
The given problem involves calculating the amount of heat released in the reaction between 40.0 mL of 1.00 M H2SO4 and 80.0 mL of 1.00 M NaOH at 20.00°C. The temperature of the solution increased to 29.20°C, and the mass of the solution is 120.0 g. The specific heat of the calorimeter and solution is 4.184 J/g•°C. To calculate the heat released in the reaction, we can use the formula q = m C_p Delta T, where q is the heat released, m is the mass of the solution, C_p is the specific heat of the solution and calorimeter, and Delta T is the change in temperature. In this case, we have the values of m, C_p, and Delta T, so we can substitute them into the formula and solve for q.
q = m C_p Delta T
q = 120.0 g x 4.184 J/g•°C x (29.20°C - 20.00°C)
q = 38.5 kJ
We will first use the given formula q = mcΔT to calculate the heat released in the reaction. We have the mass (m) of the solution as 120.0 g, the specific heat capacity (C) as 4.184 J/g°C, and the change in temperature (ΔT) as 29.20°C - 20.00°C = 9.20°C. Using these values, we get q = (120.0 g) * (4.184 J/g°C) * (9.20°C). Upon calculating, we find that q = 4620.96 J, which is equivalent to 4.62 kJ.
To know more about reaction visit :-
https://brainly.com/question/30344509
#SPJ11
What is the best explanation for the decrease in first ionization energy moving from N to O? The oxygen atom is smaller thus making it easier to remove the electrons compared with N. The electrons in N are being removed from a more stable half-full sublevel, while removing the electron from the creates a more stable half-full sublevel. The electrons in Noccupy the 2p orbitals singularly, whereasthe electrons in one of the 2p orbitals of O are paired, thus increasing the electron-electron repulsions, Moving from N to O, there are more protons in the nucleus, thus increasing the effective nuclear charge (Zeff), causing a greater amount of attraction for the valence electrons and making it harder to remove the electrons.
Answers
The best explanation for the decrease in first ionization energy moving from N to O is a combination of factors. Firstly, the oxygen atom is smaller than the nitrogen atom, making it easier to remove the electrons. Additionally, the electrons in nitrogen are being removed from a more stable half-full sublevel, while removing the electron from oxygen creates a more stable half-full sublevel. Furthermore, the electrons in nitrogen occupy the 2p orbitals singularly, whereas the electrons in one of the 2p orbitals of oxygen are paired, increasing the electron-electron repulsions. Finally, moving from nitrogen to oxygen, there are more protons in the nucleus, thus increasing the effective nuclear charge (Zeff) and causing a greater amount of attraction for the valence electrons, making it harder to remove the electrons.
For more question like first ionization energy visit the link below:
https://brainly.com/question/29629534
#SPJ11
what are some examples of long term environmental changes???
Answers
Answer: A long-term environmental change is... ice age, deforestation, urbanization, Earth's orbit, Sun's intensity, global Warming, radioactive waste/pollution. Extinction of species could happen to anyone of the food webs in a long term change.
Explanation:
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
Which two methods do scientists use to gather information?
A. Following religious beliefs
B. Observing the natural world
C. Expressing strong opinions
D. Carrying out investigations
Answers
the two methods scientist use to gather information are
. observing the natural world
. carrying out investigation
A sample of argon gas collected at a pressure of 0.918 atm and a temperature of 279 K is found to occupy a volume of 719 milliliters. How many moles of Ar gas is in the sample?
mol
Answers
A tire is at a pressure of 250 KPa at a temperature of 25°C. Calculate the pressure of the tire at -10°C.
Answers
The pressure of the tire at -10°C would be 220.64 kPa.
Pressure lawAccording to the pressure law, the pressure exerted by molecules of gases is directly proportional to the absolute temperature of the gases.
This is mathematically expressed as: P1/T1 = P2/T2
Where P1 and P2 are the initial and final pressure and T1 and T2 are the initial and final temperature of the gas.
In this case, P1 = 250 kPa, T1 = 25°C or 298K, and T2 = -10°C or 263K
P2 = P1 x T2/T1 = 250 x 263/298
= 220.64 kPa
In other words, the new pressure of the tire would be 220.64 kPa.
More on pressure law can be found here: https://brainly.com/question/1460296
#SPJ1
A very flexible helium-filled balloon is released from the ground into the air at 20 degrees celsius. The initial volume of the balloon is 5.00 L and the pressure is 760mmHg. The balloon ascends to an altitude of 20 km, where the pressure is 76.0 mmHg and the temperature is 50 degrees celsius. What is the new volume, V2, of the balloon in litres, assuming it doesn't break or leak?
Answers
To solve this problem, we can use the ideal gas law, which relates pressure, volume, and temperature of a gas. The ideal gas law is given by:
PV = nRT
where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature of the gas in Kelvin.
We can assume that the mass of the helium in the balloon remains constant, so the number of moles of helium also remains constant. Therefore, we can write:
P1V1/T1 = P2V2/T2
where P1, V1, and T1 are the initial pressure, volume, and temperature of the helium in the balloon, and P2, V2, and T2 are the final pressure, volume, and temperature of the helium at the higher altitude.
We are given:
P1 = 760 mmHg
V1 = 5.00 L
T1 = 20°C = 293 K
P2 = 76.0 mmHg
T2 = 50°C = 323 K
We can plug in these values and solve for V2:
P1V1/T1 = P2V2/T2
(760 mmHg)(5.00 L)/(293 K) = (76.0 mmHg)V2/(323 K)
V2 = (760 mmHg)(5.00 L)/(76.0 mmHg)(293 K/323 K)
V2 = 80.8 L
Therefore, the new volume of the balloon at the higher altitude is 80.8 L.
While performing experiment, we ignored the effect of the concentration of the bleach on the rate of the reaction in all four trials. Why were we able to do so?
Answers
We ignored the effect of the concentration of the bleach because:
c) We only care about the effect of the Allura Red dye on the rate of the reaction and so having excess bleach ensures the concentration of the bleach remains constant in the reaction
D) Because the bleach was in excess, the order of the reaction with respect to the bleach is pseudo-zero. The correct option is C and D.
We had the excess of the bleach in each experiment, means that the bleach concentration was constant throughout in the reaction, that allowed us to ignore the effect of the bleach concentration on the of reaction in all the four trials. This will makes sure that the reaction rate will be independent of the bleach concentration and the reaction order is the pseudo-zero with regard to the bleach.
The impact of the bleach concentration ignored on reaction rate since the variations in the bleach concentration would not be alter the rate of the reaction. Therefore, the option C and D is correct.
To learn more about bleach here
https://brainly.com/question/4617946
#SPJ4
This question is incomplete, the complete question is :
While performing this experiment, we ignored the effect of the concentration of the bleach on the rate of the reaction in all four trials. Why were we able to do so? Select all responses that apply. A) The presence of the bleach in the reaction mixture is overshadowed by the presence of the dye and so the bleach does not affect the reaction rates B) Bleach is known to be zero order for this reaction C) We only care about the effect of the Allura Red dye on the rate of the reaction and so having excess bleach ensures the concentration of the bleach remains constant in the reaction D) Because the bleach was in excess, the order of the reaction with respect to the bleach is pseudo-zero
13. If a chemist has 12.3 moles of N H 03, what is the mass of the sample?
Answers
Answer:
209.4831 g
Explanation:
number of moles × molar mass = mass of substance in g
12.3 × ( 14.0067 + 1.00484 × 3 ) = 209.4831 g
How does the proximity to water affect temperature and precipitation in the tropical, temperate, and polar zones?
written answer
science
Answers
Answer:
Water heats and cools more slowly than landmasses.
Explanation:
because the coastal regions will stay cooler in summer and warmer in winter but creating a more moderate climate with a narrower temperature range.
As the charge on the membrane of a typical neuron approaches 0 mV from -55 mV, the cell membrane is considered to have:
a. repolarized
b. hyperpolarized
c. depolarized
d. become more difficult to stimulate
e. both b and d are correct
Answers
As the charge on the membrane of a typical neuron approaches 0 mV from -55 mV, the cell membrane is considered to have depolarized.
Although the uneven distribution of lipids in membranes is now accounted for in membrane models, the influence of membrane charges on controlling the interaction of proteins with the plasma membrane is frequently disregarded. Because of the unequal distribution of charged lipids across the plasma membrane's two leaflets, the inner leaflet is negatively charged, resulting in a surface potential that attracts and binds positively charged ions, proteins, and peptide motifs.
These interactions not only produce a transmembrane potential but can also help charged membrane domains develop. To emphasize key pathways, we allude to areas outside of immunology where the effects of membrane charge are well understood. We then turn our attention to T cell receptor (TCR) signalling, reviewing the data indicating that membrane charges and membrane-associated calcium regulate phosphorylation of the TCR-CD3 complex and talking about how the immunological synapse exhibits distinctive patterns of membrane charge distribution. According to our hypothesis, when T cells are activated, charged lipids, ions in solution, and brief protein interactions create a dynamic equilibrium.
To know more about cell membrane click here:
https://brainly.com/question/12476894
#SPJ4
which element has the highest ionization energy in period 3
Answers
After considering the given the data we conclude that the ionization energy generally increases from left to right across a period. Therefore, the element with the highest ionization energy in period 3 would be located on the right side of the periodic table.
We can also see from the search results that helium has the highest ionization energy of all the elements, while sodium has the lowest ionization energy in period 3. Therefore, we can conclude that the element with the highest ionization energy in period 3 is located to the right of sodium.
Based on the periodic table, we can see that the elements in period 3 are:
Sodium (Na)
Magnesium (Mg)
Aluminum (Al)
Silicon (Si)
Phosphorus (P)
Sulfur (S)
Chlorine (Cl)
Argon (Ar)
Therefore, the element with the highest ionization energy in period 3 is most likely Argon (Ar), which is located on the far right side of the period.
In summary, the element with the highest ionization energy in period 3 is most likely Argon (Ar).
To learn more about periodic table
https://brainly.com/question/25916838
#SPJ4
how many electrons occupy the bonding molecular orbital for the h2 molecule?
Answers
In the \(H_2\) molecule, there are two electrons that occupy the bonding molecular orbital.
The \(H_2\) molecule consists of two hydrogen atoms, each containing one electron. When the two hydrogen atoms come together to form a molecule, their atomic orbitals combine to form molecular orbitals. In the case of \(H_2\), a bonding molecular orbital is formed by the constructive interference of the atomic orbitals.
This bonding orbital is lower in energy compared to the individual atomic orbitals, and it can accommodate two electrons with opposite spins due to the Pauli exclusion principle. Therefore, both electrons from the hydrogen atoms occupy the bonding molecular orbital in the \(H_2\) molecule. These two electrons contribute to the stability of the molecule by sharing the electron density between the two nuclei, resulting in a covalent bond. The occupancy of the bonding molecular orbital with two electrons ensures the formation of a stable \(H_2\) molecule.
Learn more about Pauli exclusion principle here:
https://brainly.com/question/11898781
#SPJ11
Balance the chemical equation. Values may be used more than once.
Ga + H2SO4 ⇒ Gaz(SO4)3 + H2
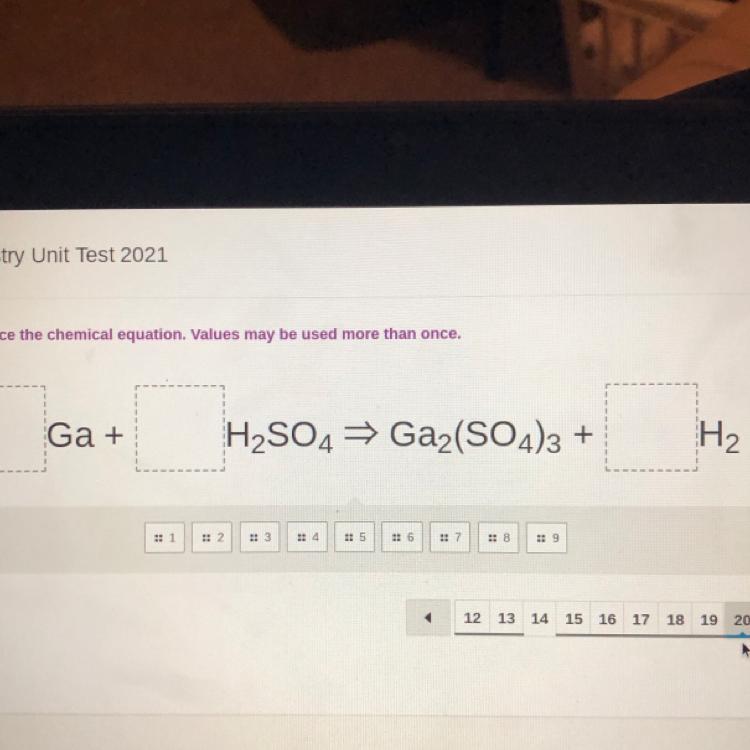
Answers
\(\\ \sf\longmapsto Ga+H_2SO_4\longrightarrow Ga_2(SO_4)_3+H_2\)
Balanced equation:-
\(\\ \sf\longmapsto 2Ga+3H_2SO_4\longrightarrow Ga_2(SO_4)_3+3H_2\)
On reactant side
Ga=2H=6SO_4=3On products side
Ga=2H=6SO_4=3Hence balanced
The pitch of a sound is related to its
frequency
amplitude
Answers
Answer:
The pitch of a sound is related to its frequency.
Explanation:
The higher the frequency, the higher the pitch will be
Amplitude is related to how loud a sound is
Determine the partial pressure and number of moles of each gas in a 15.75-L vessel at 30.0 C containing a mixture of xenon and neon gas only.
Answers
The Partial pressure of Xe and Ne will be 4.95 atm and 1.55 atm. The number of moles of Xe and Ne will be 3.13 and 0.981
Computation of partial pressure and number of moles:
Let the total pressure of the vessel= 6.5 atm and mole fraction of Xenon= 0.761
As we know,
\(\chi_{Ne} + \chi_{Xe} = 1\\\chi_{Ne}= 1- 0.761\\\chi_{Ne}= 0.239\)
According to Dalton's Law of partial pressure-
\(P_i=\chi_i\times P_{total}\)
Where,
\(P_i=\)The pressure of the gas component in the mixture
\(\chi_i=\) Mole fraction of that gas component
\(P_t=\) The total pressure of the mixture
\(P_{Xe}=(0.761)\times(6.5)\\P_{Xe}= 4.95 atm\\\\\\P_{Ne}=(0.239)\imes (6.5)\\P_{Ne}= 1.55 atm\)
Calculation:
To calculate the number of moles,
PV=nRT
\(n=\frac{PV}{RT}\)
\(n_{Xe}= \frac{4.95\times 15.75}{0.0821\times303 }\\ n_{Xe}= \frac{77.96}{24.87} \\n_{Xe}= 3.13\,mole \\\\\\n_{Ne}= \frac{1.55\times 15.75}{0.0821\times303 }\\\\n_{Ne}=\frac{24.41}{24.87}\\ n_{Ne}=0.981 \,mole\)
Learn more about Dalton's Law of partial pressure here:
https://brainly.com/question/14119417
#SPJ4