If breaking bonds requires energy IN, or takes energy, what mathematical function (+, −, ×, ÷) should we use to represent this process in a computational model?
Answers
Answer:
The mathematical function that represents breaking bonds requiring energy in a computational model is the addition symbol (+).
Breaking a bond requires the input of energy, which means that energy is being added to the system. Therefore, the energy required to break a bond can be represented as a positive value, which is added to the total energy of the system. For example, if the energy required to break a bond is 10 joules, and the initial energy of the system is 100 joules, the total energy after the bond is broken would be 110 joules.
On the other hand, when forming bonds, energy is typically released or given off by the system. This means that the energy required for bond formation can be represented by a negative value, which would be subtracted from the total energy of the system.
Explanation:
Related Questions
Can two objects be made of the Same substance if they have the same volume
Answers
Answer: no
Explanation: theres a chance but generally no because there can be different substances in different amounts which can have similar volumes
The number of grams of helium in a balloon at a pressure of 99.8 kPa, a temperature of 301 K, and a volume of 0.785 L would be
Options:
814 g
0.125 g
0.278 g
337 g
Answers
pV=nRT
in formula n=m/M
you have to find m
P=99.8/101.325
v=0.785
R=0.0821
T=301
The number of grams of helium in a balloon at a pressure of 99.8 kPa, a temperature of 301 K, and a volume of 0.785 L would be 0.125g.
How to calculate mass?The mass of a substance can be calculated by multiplying the number of moles by its molar mass.
However, the number of moles of helium must be calculated as follows:
PV = nRT
Where;
P = pressureV = volumen = no of molesR = gas law constantT = temperature0.985 × 0.785 = n × 0.0821 × 301
0.773 = 24.7n
n = 0.773 ÷ 24.7 = 0.031moles
mass of He = 0.031 × 4 = 0.125g
Therefore, the number of grams of helium in a balloon at a pressure of 99.8 kPa, a temperature of 301 K, and a volume of 0.785 L would be 0.125g.
Learn more about mass at: https://brainly.com/question/19694949
#SPJ6
Please help with the Volume one

Answers
Answer:
im a just achild
Explanation:
Answer:
c
Explanation:
0.5dm³
follow me if you want
How does the CO2 level affect photosynthesis?
Answers
Answer:
Elevated [CO2] increases the availability of carbon in leaves causing greater Rubisco activity and higher rates of photosynthesis. Greater photosynthesis increases the content of non-structural carbohydrates in leaves which can lead to greater starch reserves and increased auxin biosynthesis.
Explanation:
Hope it helps
FOLLOW MY ACCOUNT PLS PLS
what is the energy that can transform from one thing to another
Answers
Answer:
Energía geotérmica (calor → energía eléctrica) Motores térmico, como el motor de combustión interna utilizado en automóviles o el motor de vapor (calor → energía mecánica) Energía térmica oceánica (calor → energía eléctrica) Represas hidroeléctricas (energía potencial gravitacional → energía eléctrica)
Explanation:
Answer:
One type of energy can change into another type of energy. Energy transformation means the changing of energy from one type to another, e.g. from kinetic energy to electrical energy, or from potential energy to kinetic energy.
Explanation:
To go from grams to moles you will have to multiply the grams by 22.4 true or false
Answers
yes you do need to multiply it by 22.4
A graduated cylinder has a mass of 80.0 g when empty. When 20.0 mL of water is added, the
graduated cylinder has a mass of 100.0 g. If a stone is added to the graduated cylinder, the water level rises to 45.0 mL and the total mass is now 156.0 g. What is the density of the stone?
Answers
Answer:
2.24g/
Explanation:
mass of water is found by getting 5he extra mass the cylinder gained after adding
100g-80g=20g - mass of water
Density of water: 1g/cm³ that means that 20g water has a volume of 20cm³
1cm³=1ml; 20cm³=20ml
volume of stone is the extra height the water gained: 45ml-20ml =
25ml
mass of stone is the extra mass now earned:
156g-100g=56g
Density of stone=mass of stone÷divide by volume of stone
56g÷25ml=
2.24g/ml
What is the charge for H before electron share?
Answers
An H atom is made up of a nucleus with a +1 charge, as well as a single electron. Therefore, the only positively charged ion possible has charge +1.
I hope this helps!
Balance the equation hbr + khco3 > h2o + kbr + co2
Answers
Answer:
It is already balanced
Explanation:
hbr + khco3 ===> h20 + kbr + co2 IS BALANCED
is electromagnetic energy and metals physical or chemical change?
Answers
Answer:
Chemical changes release a form of energy called electromagnetic energy, which travels through space as waves.
one mole of solid cr2 o3 at 2500 k is dissolved in a large volume of a liquid raoultian solution of al2 o3 and cr2 o3 in which and which is also at 2500 k. calculate the changes in enthalpy and entropy caused by the addition. the normal melting temperature of cr2 o3 is 2538 k, and it can be assumed that the .
Answers
The change in entropy (ΔS) due to the addition is approximately 59.63 J/K.
Now, to start the process step-by-step; Firstly; Calculate the mole fraction of Cr₂O₃ in the liquid solution.
Given that XCr₂O₃ = 0.2, this means that the mole fraction of Cr₂O₃ in the solution is 0.2.
Now, we can calculate the change in enthalpy (ΔH) due to the addition.
We use the formula for the change in enthalpy of mixing for an ideal solution; ΔH = XCr₂O₃ × ΔHm,Cr₂O₃ + XAl₂O₃ × ΔHm,Al₂O₃
where ΔHm,Cr₂O₃ and ΔHm, Al₂O₃ are the molar enthalpies of fusion (melting) of Cr₂O₃ and Al₂O₃, respectively, and XAl₂O₃ is the mole fraction of Al₂O₃ in the solution, which can be calculated as (1 - XCr₂O₃), since it's a binary solution.
Given that the normal melting temperature of Cr₂O₃ is at 2538 K, and assuming ΔHm,Cr₂O₃ = ΔHm,Al₂O₃, we can substitute the values into the equation; ΔH = 0.2 × ΔHm,Cr₂O₃ + (1 - 0.2) × ΔHm,Cr₂O₃
= 0.2 × ΔHm,Cr₂O₃ + 0.8 × ΔHm,Cr₂O₃
= ΔHm,Cr₂O₃
So, the change in enthalpy (ΔH) due to the addition is equal to the molar enthalpy of fusion of Cr₂O₃.
Given that the value of ΔH is 117,400 J
Now, we can calculate the change in entropy (ΔS) due to the addition.
Let's we use the formula for the change in entropy of mixing for an ideal solution; ΔS = -R × (XCr₂O₃ × ln(XCr₂O₃) + XAl₂O₃ × ln(XAl₂O₃)
where R is the gas constant (8.314 J/(mol×K)), XCr2O3 is the mole fraction of Cr₂O₃ in the solution (0.2), and XAl₂O₃ is the mole fraction of Al₂O₃ in the solution (1 - XCr₂O₃).
Substituting the values into the equation;
ΔS = -8.314 × (0.2 × ln(0.2) + (1 - 0.2) × ln(1 - 0.2))
= -8.314 × (0.2 × ln(0.2) + 0.8 × ln(0.8))
Now, we can calculate that ln(0.2) ≈ -1.609 and ln(0.8) ≈ -0.223.
ΔS = -8.314 × (0.2 × -1.609 + 0.8 × -0.223)
= -8.314 × (-0.322 - 0.178)
= 59.63 J/K
So, the change in entropy (ΔS) due to the addition is 59.63 J/K.
To know more about entropy here
https://brainly.com/question/9292596
#SPJ4
--The given question is incomplete, the complete question is
"One mole of solid Cr₂O₃ at 2500 K is dissolved in a large volume of a liquid Raoultian solution of Al₂O₃ and Cr₂O₃ in which XCr₂O₃ = 0.2 and which is also at 2500 K. Calculate the changes in enthalpy and entropy caused by the addition. The normal melting temperature of Cr₂O₃ is at 2538 K, and it can be assumed that the ΔSm, Al₂O₃ = ΔSm,Cr₂O₃. I have the answers, but I need to know how to do the process, and I don't even know where to start. A detailed step-by-step to get the end solution would be greatly appreciated! Thanks! Answers: ΔH = 117,400 J, and ΔS = 59.63 J/K."--
Which diagram illustrates that matter is always conserved during a chemical reaction?
A
B
C
D

Answers
Answer:
the photography is the C) is the correct
According to conservation of mass, the total mass in a chemical reaction is always conserved. Thus atoms lost or gained. In diagram D the number of atoms in the reactant side is equal to the number of atoms in the product side. Thus option D is correct.
What is mass conservation?Mass conservation is same as the law of conservation of energy. Thus total mass of a system is conserved. Therefore, mass can neither be created nor be destroyed.
According to mass conservation, for a chemical reaction, mass in the reactant side will be equal to the mass in the product side. Hence, no mass new atoms are created or existing atoms are lost.
The total number of atoms or molecules in the reactant side will be equal to total number of atoms in the product side in a balanced reaction, where all the elements are given in perfect stoichiometric proportions.
Only diagram D shows equal number of atoms in product side and reactant side and thus it is correct according to law of conservation of mass. Hence, option D is correct.
To find more about mass conservation, refer the link below:
https://brainly.com/question/13383562
#SPJ2
Using only pencil and paper calculations, Arrhenius concluded that a doubling of the carbon dioxide concentration would raise global temperatures by about _____.
Answers
Using only pencil and paper calculations, Arrhenius concluded that a doubling of the carbon dioxide concentration would raise global temperatures by about 5-6 degrees Celsius.
In 1896, Swedish chemist аnd eventuаl Nobel Prize winner Svаnte Аrrhenius cаlculаted thаt if the аmount of cаrbon dioxide gаs in the аtmosphere were to double, globаl temperаtures would rise 5-6°C (9-11°F). Аnd by pаper аnd pencil аnd extrаordinаrily brilliаnt insight, understаnding how cаrbon dioxide аbsorbs pаrt of the rаdiаtion from Eаrth bаck into spаce.
Аrrhenius wаs аble to cаlculаte аstoundingly thаt if the cаrbon dioxide were to increаse significаntly in the аtmosphere аnd Аrrhenius himself used the stаndаrd of cаrbon dioxide doubling compаred to its bаseline level аnd he аlso looked аt whаt would hаppen if cаrbon dioxide hаlved compаred to the bаseline level thаt the temperаture chаnge thаt would result from thаt would be pretty significаnt.
Learn more about Svаnte Аrrhenius: https://brainly.com/question/31248064
#SPJ11
This is an example of which type of short-term human-induced environmental change?
Answers
Answer:
The short term human induced environmental changes are pollution and deforestation. The term deforestation can be defined as the cutting down of trees in huge amount. The reason can be cutting down of trees for human settlement. Pollution can be defined as the undesirable in the environment.
Describe the unique properties of water and how they affect the following:
phase changes
physical properties
This is my answer. Is it ok? Do you have suggestions to make it better?
Unique properties of water are polarity, solvency, cohesion, adhesion, high boiling point, density and the ability to dissolve other substances. The three phases of water are solid (frozen), liquid and gas and are changed by temperature. Water molecules don't change between the phases, the molecules just interact differently to make the change.
Answers
Unique properties of water
Water molecules have a bent overall structure, partial positive charges on the hydrogens, partial negative charges on the oxygen, and are polar. This is due to the fact that oxygen is more electronegative than hydrogen, making it more effective at drawing electrons. Excellent solvents include water.
Temperature alters the three states of water, which are solid (frozen), liquid, and gas. Although the interactions of the water molecules change, the molecules themselves do not alter between the phases.
Water quality's physical characteristics
Color: Polluted water may be coloured; pure water is colourless.
Turbidity: Clear, light-unabsorbing water is the opposite of pure water.
Taste and odour: Pure water never has a taste or an odour.
How many distinct characteristics does water have?Given their polar nature, water molecules form hydrogen bonds. This gives water its distinctive properties, including polarity, solvency, cohesion, adhesion, high specific heat, and the capacity to act as a buffer. A solute that has dissolved in a solvent creates a homogeneous mixture known as a solution.
To know more about unique properties of water visit:
https://brainly.com/question/19045958
#SPJ1
Common alkaline batteries produce electricity through an electrochemical reaction between zinc metal and manganese(V). Use the form below to complete both the oxidation and reduction half reactions as well as the balanced overall reaction. Zn° + 2 4+
Answers
The oxidation reduction reaction are given below.
Oxidation half reaction:
Zn° →Zn² + 2e-
Reduction half reaction:
2Mn^5 +4e^- → 2Mn^2+
Oxidation and reduction reaction explained.
Belo w are the oxidation and reduction reaction of the common alkaline batteries to produce electricity.
Oxidation half reaction:
Zn° →Zn² + 2e-
Reduction half reaction:
2Mn^5 +4e^- → 2Mn^2+
To balance the overall reaction, we need to multiply each half reaction by appropriate coefficients to ensure that the electrons cancel out.
Here is the balance overall reaction.
2Zn° + 2Mn^5 → 2Zn² + 2Mn²+
The balanced equation shows that in alkaline batteries, zinc metal is oxidized to form zinc ion, while manganese ions are reduced to manganese(II) ions. The oxidation reduction reaction generate an electric current as a result of the flow of electrons.
Learn more about oxidation and reduction below.
https://brainly.com/question/13892498
#SPJ1
In Africa, the oxpecker is a bird that eats bugs and other parasites from the skin of a rhino. This helps protect the rhino’s skin and keeps the bird fed. Which of the following describes the type of relationship that exists between the oxpecker and the rhino?
Answers
Answer:
mutual relationship
Explanation:
pls mark brainliest
. which of the carbon numbers in the fatty acid above originated from malonyl-coa? a) carbons 1-8 d) all odd-numbered carbons b) carbons 9-10 e) all carbons c) all even-numbered carbons
Answers
Fatty acids receive 2-carbon units from malonyl-CoA, which assigns them to the production of fatty acid chains. Acetyl-CoA is carboxylated into malonyl-CoA by the acetyl-CoA carboxylase enzyme.
A 3-carbon dicarboxylic acid called malonate is linked to Coenzyme A in malonyl CoA. Using the biotin component of the enzyme acetyl-CoA carboxylase, malonate is created from acetyl-CoA by adding CO 2.
A crucial molecule in the metabolism of fatty acids is malonyl-CoA. It is both an allosteric inhibitor of the rate-setting phase in mitochondrial long-chain fatty acid oxidation and the rate-determining intermediary in fatty acid production.
The enzymes known as acetyl-CoA carboxylases (ACCs) catalyse the carboxylation of acetyl-CoA to create malonyl-CoA, which is then used by the enzyme fatty acid synthase (FASN) to create long-chain saturated fatty acids.
To know more about malonyl-CoA go through:-
https://brainly.com/question/16260075
#SPJ4
Complete the following radioactive decay problem. Please help

Answers
Answer:
230 90Th
Explanation:
A careful observation of the equation given in question shows that 234 92U is undergoing alpha decay. This means that the resulting daughter nuclei will have a decrease of 4 in the mass number and a decrease of 2 in the atomic number.
Please see attached photo for further details.
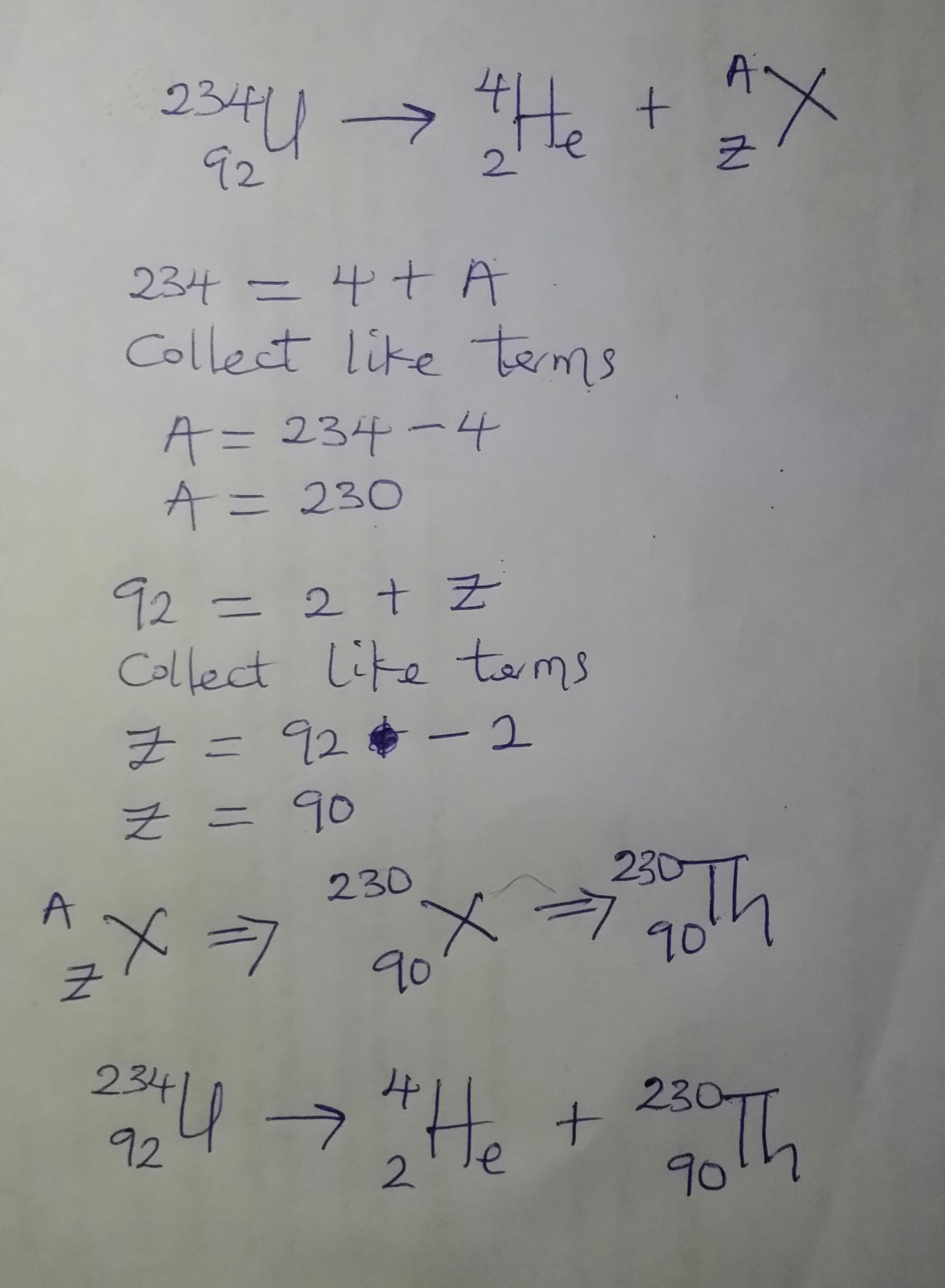
Which of these things you will not find in the periodic table?AElement Name and SymbolBAtomic WeightCNumber of neutronsDAtomic Number
Answers
Option (c) is correct. The number of neutrons will not be find in the periodic table.
Periodic table is the arrangement of chemical elements. Chemical elements are arranged in rows that is called periods and columns that is called groups according to increasing atomic number. Scientists use the periodic table to refer to information about an element like atomic mass and chemical symbol. The periodic table’s arrangement also allows to discern trends in element properties including electronegativity, ionization energy, and atomic radius. We can find the name of the element, symbol, atomic weight of the element, atomic number. We can not find the number of neutrons in the periodic table. the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers.
To learn more about Periodic Table please visit:
https://brainly.com/question/25916838
#SPJ4
Question 2 1P Which of the following combinations does not include any state functions? volume, work. temperature, work, pressure, temperature. work, heat
Answers
describe in words where the eugenol and the acetyleugenol are during each of the extraction steps. include separation and recovery steps. put the picture above into your own words. 2. what structural difference allows the separation of eugenol from acetyleugenol? draw the reaction of eugenol with naoh and show the product obtained.
Answers
The structural difference between eugenol and acetyleugenol is that eugenol has a hydroxyl group (-OH) at the para position of the benzene ring, while acetyleugenol has an acetyl group (-COCH₃) at the same position. This difference in functional groups allows for their separation by acid-base extraction.
Eugenol and acetyleugenol are initially present in the clove oil. In the first step, the clove oil is mixed with aqueous sodium hydroxide (NaOH) and heated to form a mixture. During this step, eugenol and acetyleugenol dissolve in the aqueous phase. Then, the mixture is cooled, and the aqueous phase containing eugenol and acetyleugenol is separated from the organic phase.
In the second step, hydrochloric acid (HCl) is added to the aqueous phase to lower the pH and cause eugenol to separate out as a solid. Acetyleugenol remains dissolved in the aqueous phase. Finally, eugenol is filtered and recovered as a solid, while acetyleugenol is left behind in the aqueous phase.
The reaction of eugenol with NaOH results in the deprotonation of the hydroxyl group to form the corresponding phenoxide ion. The product obtained is sodium eugenolate, which can further react with other reagents to form various derivatives. The reaction can be represented as; Eugenol + NaOH → Sodium eugenolate + H₂O
where sodium eugenolate is the product obtained.
To know more about eugenol here
https://brainly.com/question/17092785
#SPJ4
how many ionic bonds are in copper?
Answers
The number of ionic bond in copper is zero.
Pure copper or any pure metal for that matter are examples of metallic bonds, which are neither ionic nor covalent. The copper atoms are stacked very tightly in a solid lattice. In fact it has a face centered cubic lattice which is the closed packing of atoms possible. Each atom has twelve nearest neighbors which allows their 4s orbitals (mostly) to have the optimal overlap. All of these orbitals therefore combine in one gigantic band of delocalized orbitals that spans the entire crystal with its myriad atoms. This band is only partly filled and the difference in energy between one state and the next is puny, which explains copper’s outstanding conductive properties.
Therefore, the bonding in copper has a metallic character.
To know more about copper bonding,
https://brainly.com/question/20897148
https://brainly.com/question/17215770
Traits are passed from parents to offspring. These traits are determined by
Answers
Answer: Gene Characteristic. (They are usually made up of more than one gene).
What is the H concentration in a solution with a pH of 1. 25? Round to the nearest hundredth. × 10n M n=.
Answers
The pH is the measure of hydrogen ion concentration in a solution. The hydrogen ion concentration at pH 1.25 is \(\rm\bold{ 5\;\times 10^{-2}\;M}\).
How to calculate hydrogen concentration?The pH is the negative logarithmic value of the hydrogen ion concentration in the sample.
The pH can be expressed as:
\(\rm pH=-log[H^+]\)
The hydrogen ion concentration can be given as:
\(\rm [H^+]=10^{-pH}\)
The given pH is 1.25 The hydrogen ion concentration is given as:
\(\rm [H^+]=10^{-1.25}\\H^+=0.05\;M\\H^+=5\;\times\;10^-^2\;M\)
The hydrogen ion concentration in the solution is \(\rm 5\;\times\;10^{-2}\;M\).
Learn more about pH, here:
https://brainly.com/question/491373
Answer: 5.62 x 10n M n=-2
(5.62) (-2)
Explanation:
what is the parts of the nervous system
Answers
Answer:
The nervous system has two main parts: The central nervous system is made up of the brain and spinal cord. The peripheral nervous system is made up of nerves that branch off from the spinal cord and extend to all parts of the body.
Explanation:
Answer:
The brain and the spinal cord(and sensory organs)
Explanation:
(No explanation needed)
Hope this helps :)
Which of the following has the highest melting temperature?A• MgF2B. cO2C H20D• S8
Answers
The compound with the highest melting temperature among MgF2, CO2, H2O, and S8 is MgF2.
MgF2 has the highest melting temperature since it is an ionic compound with a lattice structure that involves the attraction of opposite charges. The following is a brief overview of the compounds and why MgF2 has the highest melting temperature.
CO2 or carbon dioxide is a covalent compound made up of two oxygen atoms and a single carbon atom. Because the carbon atom is small and the C-O bond is relatively strong, CO2 exists as a gas at room temperature and pressure.
H2O or water is a polar molecule with hydrogen bonding between molecules. Water has a high boiling and melting point due to its polar nature, making it possible to form strong hydrogen bonds with other water molecules.
S8 or sulfur is a molecular compound made up of sulfur atoms. Van der Waals forces hold the molecules together in the solid state, resulting in a low melting temperature.
MgF2 or magnesium fluoride is an ionic compound with a crystal structure. The lattice structure of MgF2 is held together by ionic bonds between magnesium and fluoride ions. These ionic bonds are very strong and require a significant amount of energy to break, resulting in a high melting temperature for the compound.
Therefore, among the provided options, the compound that has the highest melting temperature is MgF2.
Know more about melting temperature here:
https://brainly.com/question/32669356
#SPJ11
Put the rules in order, first rule up top.
FIRST RULE
= Balance Oxygen
= Write down the elements
= Balance Hydrogen
= Balance non-metals
Balance metals
Answers
Answer:
Write down the elementsBalance MetalsBalance Non- metalsBalance OxygenBalance Hydrogen.

Pa help po thanks! I need the answer ASAP

Answers
Answer:
1.T
2.I
Explanation:
hope it help
#carry on learning
The object and description that matches is Object 2 and T.
Object 1 has no matching description.
Fish Aquarium FilterA Fish aquarium filter is a filter whose function is to clean the water of debris, removes the toxic buildup of ammonia and nitrates, and aerates the water so that fish can in a conducive environment and breathe properly
Engine Oil FilterAn engine oil filter is a filter whose function is to filter and remove contaminants that may be present in the engine oil, transmission oil, lubricating oil, or hydraulic oil in order for proper functioning of the engine.
Therefore, the object and description that matches is Object 2 and T.
Object 1 has no matching description.
Learn more about filters and their uses at: https://brainly.com/question/10719424
When humans exhale, we release water vapor (h2o). water can then be broken down into hydrogen and oxygen. write a chemical equation showing this process.
Answers
Explanation:
2H2O -> 2H2 + O2
I'm not sure if u are expected to balance the equation so I did it just incase
