If we project the relation r of problem 3 onto s(a, c, e), what nontrivial fd’s and mvd’s hold in s?
Answers
To determine the nontrivial FD's and nontrivial MVD's in s(a, c, e) upon projecting relation r of problem 3 onto it, the main answer will be as follows:
Given: Relation r of problem 3:(a, b, c, d, e, f)ABCD → EFDE → AFD → C Nontrivial FD's and MVD's in s:(a, c, e)
Let's consider the projections of each of the FD's and MVD's present in the relation r of problem 3 onto the relation s(a, c, e).FD: A → E
Upon projecting FD A → E of relation r onto s(a, c, e), we get the following FD in s:(a) → (e)FD: E → A
Upon projecting FD E → A of relation r onto s(a, c, e), we get the following FD in s:(e) → (a)FD: C → Null
Upon projecting FD C → Null of relation r onto s(a, c, e), we get the following FD in s:(c) → NullMVD: AB → CDMVD AB → CD of relation r can be represented as follows:AB → C and AB → D
Upon projecting this MVD of relation r onto s(a, c, e), we get the following MVD in s:(a, b) → c and (a, b) → d
Thus, the nontrivial FD's and MVD's that hold in s(a, c, e) upon projecting relation r of problem 3 onto it are:(a) → (e)(e) → (a)(c) → Null(a, b) → c and (a, b) → d.
to know more about nontrivial FD's visit:
brainly.com/question/32261943
#SPJ11
Related Questions
PLEASE WILL GIVE BRAINLY IF I GET IT CORRECT

Answers
Answer:
Inferred that fossils were once part of living animals.
Explanation:
He realized that seashells that were from rocks were identical to ones on a beach.
Hope this helps. Plz mark as brainliest!
Have a great day and plz follow me! :)
Give lewis dot structures and sketch the shapes of the following: a. secl4 b. i3 - c. pscl3 (p is central) d. if4 - e. ph2 - f. tef4 2- g. n3 - h. seocl4 (se is central) i. ph4
Answers
We structure the Lewis points and outline the forms of the following elements:
For a. SeCl4, the Lewis dot structure is:
Se | Cl
= Cl | Cl
It has a tetrahedral shape with Se as the central atom.
For b. I3-, the Lewis dot structure is:
I |
- I | I
It has a trigonal planar shape with I as the central atom.
For c. PSCl3, the Lewis dot structure is :
P | Cl
- - Cl | Cl Cl
It has a trigonal pyramidal shape with P as the central atom.
For d. IF4-, the Lewis dot structure is :
I | F
- - F | F F
It has a square planar shape with I as the central atom.
For e. PH2-, the Lewis dot structure is :
P | H
- H | H
It has a bent shape with P as the central atom.
For f. TeF42-, the Lewis dot structure is :
Te | F
- - F | F F
It has an octahedral shape with Te as the central atom.
For g. N3-, the Lewis dot structure is:
N |
- N | N
It has a linear shape with N as the central atom.
For h. SeOCl4, the Lewis dot structure is:
Se | O | Cl
- - - Cl | Cl Cl
It has a tetrahedral shape with Se as the central atom.
For i. PH4+, the Lewis dot structure is:
P | H
- H | H H | H
It has a tetrahedral shape with P as the central atom.
Learn more about lewis structures:
https://brainly.com/question/20300458
#SPJ4
Why does the angle of the sun at noon, seem to change at different moths throughout the year?
Answers
Answer:
because during different seasons the earth is closer or farther from the sun
Explanation:
the earths rotation around the sun is an oval with the winter season being the closest to the sun and the summer season being the farthest
Can someone help me with my stoichiometry homework, it is due tonight.

Answers
Answer:
1. 1 mole of NaCl.
2. 1 mole of H₂O.
Explanation:
The balanced equation for the reaction is given below:
HCl + NaOH —> NaCl + H₂O
From the balanced equation above,
1 mole of HCl reacted with 1 mole of NaOH to produce 1 mole of NaCl and 1 mole of H₂O.
Next, we shall determine the limiting reactant. This can be obtained as follow:
From the balanced equation above, we can see that 1 mole of HCl reacted completely with 1 mole of NaOH. Therefore, HCl is the limiting reactant and NaOH is the excess reactant since we have 3.6 moles of NaOH.
1. Determination of the amount of NaCl produced from the reaction.
NOTE: The limiting reactant is used to determine the maximum amount produced since all of it is consumed in the reaction.
The limiting reactant is HCl and the amount of NaCl produced can be obtained as follow:
From the balanced equation above, we can see that 1 mole of HCl reacted to 1 mole of NaCl.
Thus, 1 mole of NaCl was produced.
2. Determination of the amount of H₂O produced from the reaction.
From the balanced equation above, it is evident that 1 mole of HCl reacted to produce 1 mole of H₂O.
Thus, 1 mole of H₂O was produced.
Which of the following statements correctly describe wave-particle duality? Select all that apply.
All matter exhibits wavelike motion.
Matter and energy are different forms of the same entity.
Energy and mass can be interconverted.
Answers
Wave-particle duality is a fundamental concept in quantum mechanics that describes the dual nature of particles and waves. The following statements correctly describe wave-particle duality:
1. All matter exhibits wavelike motion: This statement reflects the wave nature of particles. Even though particles have localized positions, they also exhibit wave-like properties, such as diffraction and interference.
2. Matter and energy are different forms of the same entity: According to Einstein's theory of relativity (E=mc²), energy and mass are interconnected. This concept suggests that matter can be viewed as a form of energy.
3. Energy and mass can be interconverted: This statement is a direct implication of Einstein's famous equation. It means that mass can be converted into energy and vice versa. This phenomenon is observed in nuclear reactions and particle interactions.
Wave-particle duality highlights the wave-like and particle-like behaviors exhibited by particles at the microscopic level. It revolutionized our understanding of the fundamental nature of matter and laid the foundation for quantum mechanics.
To know more about Wave-Particle Duality theory, visit:
brainly.com/question/10260414
#SPJ11
Solving for TIME: You need to get to class, 200 meters away, and you can only
walk in the hallways at about 2 m/s. (if you run any faster, you'll be caught for
running). How much time will it take to get to your class?
Answers
Answer:
1.66 mins
Explanation:
Distance = 200 m
Speed = 2 m/s
Time taken = \(\frac{Distance}{Speed} = \frac{200}{2} = 100\) secs = 1.66 mins
Draw a graph of number of electrons in the halogen molecule against the boiling point of the halogen. Label each point in your graph with the formula halogen and molecule. Can you see a trend. Make notes on your observation, draw a horizontal line in your graph to show room temperature.

Answers
As we travel down in the group, the halogens' boiling points rise. The resultant molecules may have a single carbon atom or as many as a million.
What relationship exists between the boiling point of a halogen and the amount of electrons in its molecule?The attractive force grows as the number of electrons decreases in the group, and more energy is needed to counteract these forces, raising the boiling point.
What is the pattern of halogens' boiling points?Fluorine's boiling point of -188°C, Chlorine's of -34.6°C, Bromine's of 58.8°C, and iodine's of 184°C, as well as the trend in melting temperatures, are explained by the strengthening intermolecular interactions that bind the halogen molecules together.
To know more about halogens visit:-
brainly.com/question/11156152
#SPJ1
Question 1-14
How does the carbon cycle support the Law of Conservation of Mass?
O The total amount of carbon decreases, as it moves through the carbon cycle.
O
The total amount of carbon does not change as it moves through the carbon cycle.
O The total amount of carbon gradually increases, as it moves through the carbon cycle.
The total amount of carbon changes depending on where it's located in the carbon cycle.
Answers
According to law of conservation of mass, the carbon cycle supports the law of conservation of mass as the total amount of carbon does not change as it moves through the carbon cycle.
What is law of conservation of mass?
According to law of conservation of mass, it is evident that mass is neither created nor destroyed rather it is restored at the end of a chemical reaction .
Law of conservation of mass and energy are related as mass and energy are directly proportional which is indicated by the equation E=mc².Concept of conservation of mass is widely used in field of chemistry, fluid dynamics.
Learn more about law of conservation of mass,here:
https://brainly.com/question/15289631
#SPJ9
What is the pH of a solution made by dissolving 0.00135 moles of KOH in enough water to make 2.7 liters of solution Use two decimal places when reporting your pH. For example For example: 11.91 or 3.57 etc Please show work
Answers
The pH of a solution made by dissolving 0.00135 moles of KOH in water to make 2.7l of solution is 10.70.
How to find the pH of a solution?To find the pH of a solution made by dissolving 0.00135 moles of KOH in 2.7 liters of water, follow these steps:
1. Calculate the concentration of OH- ions:
Concentration of OH- ions = moles of KOH / volume of solution
= 0.00135 moles / 2.7 L
= 0.0005 mol/L (rounded to 3 decimal places)
2. Calculate the pOH of the solution:
pOH = -log10(concentration of OH- ions)
= -log10(0.0005)
= 3.30 (rounded to 2 decimal places)
3. Calculate the pH of the solution:
pH = 14 - pOH
= 14 - 3.30
= 10.70 (rounded to 2 decimal places)
The pH of the solution is 10.70.
To know more about pH of a solution:
https://brainly.com/question/17751948
#SPJ11
what is the ph of a saturated solution of ni(oh)2? ni(oh)2 has ksp = 2.0 x 10^–15A) 4.80 B) 8.90C) 5.10 D) 9.20 E) 7.00
Answers
To determine the pH of a saturated solution of Ni(OH)2 with a Ksp of 2.0 x 10^-15, we'll follow these steps:
1. Write the dissociation equation for Ni(OH)2: Ni(OH)2(s) ⇌ Ni2+(aq) + 2OH-(aq)
2. Set up the expression for Ksp: Ksp = [Ni2+][OH-]^2
3. Let x be the concentration of Ni2+ and 2x be the concentration of OH-. Then, Ksp = (x)(2x)^2.
4. Plug in the given Ksp value and solve for x: 2.0 x 10^-15 = x(2x)^2.
5. Calculate the concentration of OH- ions (2x).
6. Use the OH- concentration to find the pOH using the formula: pOH = -log[OH-].
7. Convert pOH to pH using the relationship: pH + pOH = 14.
So, the pH of the saturated solution of Ni(OH)2 is approximately 9.20 (Option D).
for more questions on pH of saturated solution: https://brainly.com/question/31325727
#SPJ11
To determine the pH of a saturated solution of Ni(OH)2 with a Ksp of 2.0 x 10^-15, we'll follow these steps:
1. Write the dissociation equation for Ni(OH)2: Ni(OH)2(s) ⇌ Ni2+(aq) + 2OH-(aq)
2. Set up the expression for Ksp: Ksp = [Ni2+][OH-]^2
3. Let x be the concentration of Ni2+ and 2x be the concentration of OH-. Then, Ksp = (x)(2x)^2.
4. Plug in the given Ksp value and solve for x: 2.0 x 10^-15 = x(2x)^2.
5. Calculate the concentration of OH- ions (2x).
6. Use the OH- concentration to find the pOH using the formula: pOH = -log[OH-].
7. Convert pOH to pH using the relationship: pH + pOH = 14.
So, the pH of the saturated solution of Ni(OH)2 is approximately 9.20 (Option D).
for more questions on pH of saturated solution: https://brainly.com/question/31325727
#SPJ11
2. Which of the following reaction sequences describes the method of determining the amount of iodate anion (IO3 ) in a saturated solution of calcium iodate (Ca(I03)2)2 1ztoq) IO;? + 6H* (aq ~ 3 1(4 3H,Od) 21 (q) + 8406 (4 1z(0q) Sz0,2 b. IO3 (44) SO,? (4) 104 (4) S0,7(4 SO37(4) = S,03 (MAli ' SO4? (4) Sz02 (4) IO; (aq) + 5 F (aq) = 6 H* (aq) 12(4q) " 3 HzO() Izwp " Sz032 (qxii -+ 2 To S,06 (4q)
Answers
The reaction sequence that describes the method of determining the amount of iodate anion (IO3) in a saturated solution of calcium iodate (Ca(IO3)2) is given .
The anion that has the formula IO3 is known as an iodate. Since it is present in the majority of ores that contain iodine, it is the most prevalent type of iodine found in nature. Iodide salts are typically colorless in appearance.
Calcium iodate has the molecular formula, Ca (IO3) 2, and the molecular weight of 389.8834 g/mol where iodine atom is in the +5 oxidation state. This composition occurs as a mineral “ Lautarite (Calcium Iodate·Monohydrate). It can be prepared by the reaction of iodic acid upon the carbonate: CaCO3 + 2HIO3 ⇒ Ca (IO3)2 + CO2 + H2O
a.
IO3-(aq) + 6H+(aq) ~ 3I2(s) + 3H2O(l)b. 2IO3-(aq) + 10Fe2+(aq) + 16H+(aq) → I2(s) + 10Fe3+(aq) + 8H2O(l)
For the determination of the amount of iodate anion (IO3-) in a saturated solution of calcium iodate (Ca(IO3)2), the second sequence, b, is used.
The sequence involves the reaction of 2 iodate anions with 10 ferrous ions and 16 hydrogen ions to yield Iodine, 10 ferric ions, and 8 water molecules.
The amount of iodate anion present can be determined by the quantity of iodine produced.
The correct option is b.
2IO3-(aq) + 10Fe2+(aq) + 16H+(aq) → I2(s) + 10Fe3+(aq) + 8H2O(l)
To know more about the iodate ion https://brainly.com/question/4910239
#SPJ11
The diagram shows a position (person) on Earth at 3:00 a.m. What is likely to happen to that position (person) in 12 hours? Select the best answer.
A. It will remain stationary.
B. It will rotate and be in the sun.
C. It will rotate and return away from the sun.
Answers
B.It will rotate and be in the sun.
The molar mass of barium nitrate (Ba(NO3)2) is 261. 35 g/mol. What is the mass of 5. 30 × 1022 formula units of Ba(NO3)2? 0. 0900 g 12. 0 g 23. 0 g 3,130 g.
Answers
Answer:
\(\boxed{\boxed {\sf 23.0 \ g \ Ba(NO_3)_2}}\)
Explanation:
We are asked to find the mass of 5.30 ×10²² formula units of barium nitrate.
1. Formula Units to MolesFirst, we convert formula units to moles using Avogadro's Number or 6.022×10²³. This is the number of particles (atoms, molecules, formula units, etc.) in 1 mole of a substance. In this case, the particles are formula units of barium nitrate.
Set up a conversion factor using Avogadro's Number.
\(\frac{6.022 \times 10^{23} \ formula \ units \ Ba(NO_3)_2}{ 1 \ mol \ Ba (NO_3)_2}\)
We are converting 5.30×10²² formula units, so we multiply by this value.
\(5.30 \times 10^{22} \ formula \ units \ Ba(NO_3)_2 *\frac{6.022 \times 10^{23} \ formula \ units \ Ba(NO_3)_2}{ 1 \ mol \ Ba (NO_3)_2}\)
Flip the conversion factor so the units of formula units of barium nitrate cancel.
\(5.30 \times 10^{22} \ formula \ units \ Ba(NO_3)_2 *\frac{ 1 \ mol \ Ba (NO_3)_2}{6.022 \times 10^{23} \ formula \ units \ Ba(NO_3)_2}\)
\(5.30 \times 10^{22} *\frac{ 1 \ mol \ Ba (NO_3)_2}{6.022 \times 10^{23}}\)
\(\frac{5.30 \times 10^{22} }{6.022 \times 10^{23}} \ mol \ Ba(NO_3)_2\)
\(0.0880106276984 \ mol \ Ba(NO_3)_2\)
2. Moles to GramsNext, convert moles to grams using the molar mass. The molar mass of barium nitrate is 261.35 grams per mole.
Set up a conversion factor using the molar mass.
\(\frac{ 261.35 \ g \ Ba(NO_3)_2} {1 \ mol \ Ba(NO_3)_2}\)
Multiply by the number of moles we calculated.
\(0.0880106276984 \ mol \ Ba(NO_3)_2 *\frac{ 261.35 \ g \ Ba(NO_3)_2} {1 \ mol \ Ba(NO_3)_2}\)
The units of moles of barium nitrate cancel.
\(0.0880106276984*\frac{ 261.35 \ g \ Ba(NO_3)_2} {1}\)
\(23.001577549 \ g \ Ba(NO_3)_2\)
If this is rounded to the tenths place, the 0 in the hundredth place tells us to leave the 0 in the tenths place.
\(23.0 \ g \ Ba(NO_3)_2\)
The mass of 5.30 ×10²² formula units of barium nitrate is approximately 23.0 grams.
Why was periodic table developed
Answers
Answer:
The periodic table was created to organize the elements and provide physical and chemical properties, also to group with elements like it.
Hope this helps
The recommended dose of aspirin will yield approximately 100.0 micrograms/mL in the blood. How many molecules of aspirin (C9H8O4) are in a drop (0.100 mL) of blood?
Answers
Answer:
3.34x10¹⁶ molecules of aspirin are in a drop of blood
Explanation:
The recomended dose of aspirin in blood is 100.0μg/mL =
1x10⁻⁴g aspirin / mL of blood.
In a drop (0.100mL) there are:
0.100mL ₓ (1x10⁻⁴g aspirin / mL of blood) = 1x10⁻⁵g aspirin.
Molecular mass of aspirin is:
9C = 12.01g/mol ₓ 9 = 108.09g/mol
8H = 1.01g/mol ₓ 8 = 8.08g/mol
4O = 16g/mol ₓ 4 = 64g/mol
108.09 + 8.08 + 64 = 180.17g/mol
Thus, moles of aspirin in 1x10⁻⁵g are:
1x10⁻⁵g ₓ (1mol / 180.17g) = 5.55x10⁻⁸ moles of aspirin
In 1 mole, you have 6.022x10²³ molecules, thus:
5.55x10⁻⁸ moles of aspirin ₓ (6.022x10²³ molecules / 1 mole ) =
3.34x10¹⁶ molecules of aspirin are in a drop of bloodWhat is the solution to the problem expressed to the correct number of significant figures?
7.21= ?
A. 1.688
OB.
1.69
O C.
1.7:
OD
1.70
Answers
Answer:
(102 900 ÷ 12) + (170 × 1.27) = 8800
Step 1. Evaluate the expressions inside the parentheses (PEMDAS)
102 900 ÷ 12 = 8575
170 × 1.27 = 215.9
In multiplication and division problems, your answer can have no more significant figures than the number with the fewest significant figures.
Thus, the underlined digits are not significant, but we keep them in our calculator to avoid roundoff error.
Step 2. Do the addition (PEMDAS).
8575
+ 215.9
= 8790.9
Everything that you add to an insignificant digit gives an insignificant digit as an answer.
Thus, the underlined digits are not significant.
We must drop them and round up the answer to 8800.
Explanation:
Which set of numbers will balance the following equations? 1's have been included for clarity.__Mn3N4 + __NaF --> __MnF4 + __Na3N a 1; 4; 1; 4 b 1; 4; 3; 2 c 1; 12; 3; 4 d 3; 2; 3; 2
Answers
ANSWER
Option C
EXPLANATION
Given that;
\(\text{ ----- Mn}_3N_4\text{ }+\text{ ---- NaF }\rightarrow\text{ ---- MnF}_4\text{ }+\text{ ---Na}_3N\)In the reaction above, we have the following data
At the reactants side;
3 atoms of manganese
4 atoms of nitrogen
1 atom of sodium
1 atom of fluorine
At the products side
1 atom of manganese
4 atoms of fluorine
3 atoms of sodium
1 atom of nitrogen
To balance the above equation, apply the law of conservation mass
Law of conservation of mass states that matter can neither be created nor destroyed but can e transformed from one formato another.
To balance the equation, 1 mole of Mn3N4 reacts with 12 moles of Na Tto give 3 moles of MnF4 and 4 moles of Na3N
So, the new equation becomes
\(\text{ Mn}_3N_4\text{ }+\text{ 12NaF }\rightarrow\text{ 3MnF}_4\text{ }+\text{ 4Na}_3N\)The following data can be deduced in the above equation
At the reactants side
3 atoms of Mn
4 atoms of N
12 atoms of Na
12 atoms of F
At the products side
3 atoms of Mn
12 atoms of F
12 atoms of Na
4 atoms of N
Looking atthe vabove data, the number of atoms of each element at the reactants side is equal to the number of atoms of same elements at the products side.
Hence, the correct answer is option Ce
u
What is the structure of a fluorine atom?
Answers
Answer:
The nucleus consists of 9 protons and 10 neutrons. Nine electrons occupy available electron shells.
WILL MARK BRAINLIEST FOR CORRECT ANSWER
Continue reading from Ruby Bridges’s autobiography.
We drove down North Galvez Street to the point where it crosses Alvar. I remember looking out of the car as we pulled up to the Frantz school. There were barricades and people shouting and policemen everywhere. I thought maybe it was Mardi Gras, the carnival that takes place in New Orleans every year. Mardi Gras was always noisy.
As we walked through the crowd, I didn’t see any faces. I guess that’s because I wasn’t very tall and I was surrounded by the marshals. People yelled and threw things. I could see the school building, and it looked bigger and nicer than my old school. When we climbed the high steps to the front door, there were policemen in uniforms at the top. The policemen at the door and the crowd behind us made me think this was an important place.
It must be college, I thought to myself.
–Through My Eyes,
Ruby Bridges
What evidence in the text supports the idea that Bridges did not realize the significance of her first day? Check all that apply.
“I remember looking out of the car as we pulled up to the Frantz school.”
“I thought maybe it was Mardi Gras.”
“As we walked through the crowd, I didn’t see any faces.”
“[The school] looked bigger and nicer than my old school.”
“The crowd behind us made me think this was an important place.”
“It must be college, I thought to myself.”
Answers
Answer: I remember looking out of the car as we pulled up to the Frantz school.”
“I thought maybe it was Mardi Gras.”
“As we walked through the crowd, I didn’t see any faces.”
“[The school] looked bigger and nicer than my old school.”
“The crowd behind us made me think this was an important place.”
“It must be college, I thought to myself.
Explanation:
“I thought maybe it was Mardi Gras.”
“[The school] looked bigger and nicer than my old school.”
“It must be college, I thought to myself.”
If I remove the table from under the book, what will happen to the book? Think about Newton’s second law of motion. Is there an acceleration? What caused the acceleration?
Answers
Newton’s second law of motion is a dot product of mass and acceleration, if you remove the table from, under the book, gravity will act on the book and pull it downwards to the centre of the earth
Newton's Second law of motion states that "the acceleration of an object depends upon two variables – the net force acting on the object and the mass of the object."
In our case the mass of the book and the force of gravity
Learn more about Newton's Laws of motion:
https://brainly.com/question/10454047
The speed of light is 2.998 x 108 m/s. What wavelength of light has a frequency of 6.71 x 1013 Hz?
Answers
Refer to the attachment

If you have an atom or copper, an ion of copper and an isotope of copper, what
can you say about all of these particles of copper?
They all have the same number of protons, 29 protons
They all have the same chemical properties
The number of protons equals the number of neutrons.
Plzz help
Answers
PLEASE HELP!! PLEASE??

What makes up soil and where do these main components come
from? ASAP
Answers
Answer:
Soil is a mixture of minerals, dead and living organisms (organic materials), air, and water.
Explanation:
Dead organisms decompose(kind of like fertilizer)
water comes from nearby watersheds, precipitation processes, etc.
Answer:
Soil is made up of three main components – minerals that come from rocks below or nearby, organic matter which is the remains of plants and animals that use the soil, and the living organisms that reside in the soil. The proportion of each of these is important in determining the type of soil that is present.
how many milligrams of sodium in a teaspoon of salt
Answers
Answer:
a SINGLE TEASPOON contains 2,300 milligrams of sodium or 2.3 grams!
Explanation:
hope this helps! :)
Question 4 "That oil sands executive is greedy and heartless and therefore can't be trusted when she claims to want to improve her company's environmental record."
O False dilemma
O Ad hominem attack
O Straw man
O Appeal to authority
Question 5 "There is no proof that humans are causing climate change so it must natural causes
O False dilemma
O Appeal to ignorance
Strawman
O Appeal to authority
Answers
That oil sands executive is greedy and heartless and therefore can't be trusted when she claims is Ad hominem attack. So, Option B is correct.
4- The argument in question 4 is an example of an ad hominem attack. This is due to the argument's focus on the character of the oil sands executuive rather than the actual problem, which is how to improve the company's environmental record.
The argument holds that the executive cannot be believed when she says she wants to improve the company's environmental record because she is avaricious and callous. This is an error in logic, though, as the executive's character may not necessarily be related to the company's environmental policies.
5- The argument in question 5 is an example of an appeal to ignorance. This is because the argument states that there is no proof that humans are causing climate change, so it must be natural causes. Just because there is no conclusive proof that humans are causing climate change, it does not mean that they are not.
The argument assumes that just because there is no evidence to the contrary, the argument must be true. This is a logical fallacy.
So, Option B is correct.
Learn more about climate change -
brainly.com/question/27170698
#SPJ11
Determine the excluded volume per mole and the volume actually occupied by a mole for a gas consisting of molecules with radius 167 pm. [Note: To obtain the volume in liters, we must express the radius in decimeters (dm).] Enter your answers in scientific notation.
Answers
Answer:
Explanation:
Volume of one mole of a gas = 22.4 litre.
No of molecules in one mole of gas = 6.02 x 10²³ .
Volume of one molecule = 4/3 π R³
= 4/3 x 3.14 x ( 167 x 10⁻¹² m )³
= 19.5 x 10⁶ x 10⁻³⁶ m³
=19.5 x 10⁻³⁰ m³
= 19.5 x 10⁻³⁰ x 10³ litre .
= 19.5 x 10⁻²⁷ litre .
So volume occupied by molecules in one mole of gas
= 19.5 x 10⁻²⁷ x 6.02 x 10²³ litre
117.4 x 10⁻⁴ litre
= .01174 litre.
Excluded volume
= ( 22.4 - .01174 ) litre .
= 22.388 litre.
What type of reaction does each of the following equations represent?
A. Single replacement
B. Double replacement
C. Decomposition
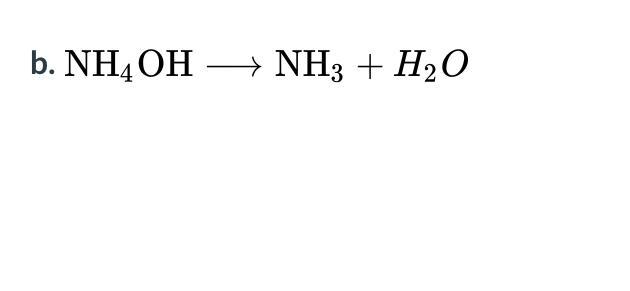
Answers
Answer:
Option C. Decomposition
Explanation:
To know which option is correct, it is important we know what is a single displacement reaction, double displacement reaction and decomposition reaction.
A Single displacement reaction can be defined as a reaction in which a single element is replaced by another element in a compound.
A double displacement reaction is a reaction involving the exchange of ions of two parts of ionic compounds to form two different compounds.
A decomposition reaction is a reaction in which a compound splits into two or more simpler compound or element.
Bearing the above definitions in mind and considering the question given above, we can conclude that the reaction given in the question above is a decomposition reaction since the compound (NH₄OH) splits into two different compound (NH₃ and H₂O)
Expressing Opinions: Compare the advantages and disadvantages of solar heating systems. What do you think their overall benefits are, compared with those of other heating systems?
Answers
Answer: Solar heating systems are energy efficient, but the amount of heating they can accomplish would not be very much in cold climates. Overall, it seems they could be put to good use in a lot of places in the world and could save a lot of energy.
Explanation:
because thats how science works ig
The spontaneous disintegration (breaking down) of a nucleus into slightly
lighter and more stable nucleus describes
A.nuclear fusion
B. nuclear fission
C. radioactive decay
D. nuclear radiation
Answers
Answer:
Radioactive Decay
Explanation:
A reaction is expected to produce 28. 3 moles of hydrogen gas. If the hydrogen is collected at 297 K and 1. 08 atm, what is the volume? 305 L H2 639 L H2 948 L H2 1,240 L H2.
Answers
To find the volume of hydrogen gas, we can use the ideal gas law equation: PV = nRT. Rearranging the equation to solve for V (volume), we have V = (nRT) / P. Given that n = 28.3 moles, R is the ideal gas constant, T is 297 K, and P is 1.08 atm, we can substitute these values to find the volume.
To determine the volume of hydrogen gas produced, we can use the ideal gas law equation, which states:
PV = nRT
Where:
P = pressure (in atm)
V = volume (in liters)
n = moles of gas
R = ideal gas constant (0.0821 L·atm/(mol·K))
T = temperature (in Kelvin)
Given:
n = 28.3 moles
T = 297 K
P = 1.08 atm
Substituting the values into the equation, we can solve for V:
V = (nRT) / P
V = (28.3 moles * 0.0821 L·atm/(mol·K) * 297 K) / 1.08 atm
V ≈ 948 L
Therefore, the volume of hydrogen gas produced is approximately 948 L.
To know more about ideal gas law click this link -
brainly.com/question/12624936
#SPJ11