Answers
Answer:
Molar mass is the mass of a given substance divided by the amount of that substance, measured in g/mol. For example, the atomic mass of titanium is 47.88 amu or 47.88 g/mol. In 47.88 grams of titanium, there is one mole, or 6.022 x 1023 titanium atoms.
Explanation:
Could I have branliest , heart, and 5 stars
Thanks!
Related Questions
a worker isolotes 2.675g of SIF4 after reacting 2.339g of SIo2 with HF what are theorical yield and actual yield?
Answers
Answer:
The actual yield is 2.675 grams and the theoretical yield is 4.0543 grams.
Explanation:
Based on the given question the reaction will be,
SiO₂ (s) + 4HF ⇒ SiF₄ + 2H₂O (l)
Let w be the concentration of silicon tetrafluoride generated by reacting silicon dioxide with hydrogen fluoride. The mass of silicon dioxide given in the question is 2.339 grams. The molar mass of silicon dioxide is 60 grams per mole and the molar mass of silicon tetrafluoride is 104 grams per mole.
Therefore, it can be said that 60 grams of silicon dioxide is giving rise to 104 grams of silicon tetrafluoride. So, 2.339 grams of silicon dioxide will generate,
As w is the weight of silicon tetrafluoride produced, so with the help of cross-multiplication we get,
60 × w = 2.339 × 104
w = 2.339 × 104 / 60 = 4.054 grams is the theoretical yield. However, the actual yield is 2.675 grams. So, the percent yield will be,
Percent yield = actual yield / theoretical yield × 100
Percent yield = 2.675 grams / 4.054 grams × 100
Percent yield = 65.98 %
The theoretical yield would be 4.052 g while the actual yield is 2.675 g
The reaction between SIO2 with HF can be represented by the following equation:
\(SiO_2 + 4HF ---> 2H_2O + SiF_4\)
The ratio of SiO2 reacted to SiF4 produced is 1:1.
Theoretically:
mole of 2.339 SiO2 = mass/molar mass
= 2.339/60.08
= 0.00389 moles
Thus, equivalent mole of SiF4 = 0.00389 moles
Mass of 0.00389 moles of SiF4 = mole x molar mass
= 0.00389 x 104.0791
= 4.052 g
Hence, the theoretical yield is 4.052 g while the actual yield is 2.675 g
More on reaction yields can be found here: https://brainly.com/question/7786567?referrer=searchResults
observe the increase in temperature every 60 seconds for 300 seconds
final temp of metal -
initial temp of water -
final temp of both -
in celsius
Answers
The final temperature of the metal is 1.88°C, while the final temperature of water is 50°C. The final temperature of both is 25.94°C (average of 1.88°C and 50°C).
To answer the question, it is necessary to use the formula: Q = m * c * ΔT. The formula is used to calculate the amount of heat gained or lost by a substance. Here, the heat lost by the metal will be equal to the heat gained by water. Let's use the following terms in our answer:
- ΔT: change in temperature
- m: mass of substance
- c: specific heat capacity
Initially, the metal has a higher temperature, while the water has a lower temperature. Heat energy will flow from the metal to the water until both substances reach a common temperature.
The rate of heat transfer will be directly proportional to the difference in temperature between the two substances. To observe the increase in temperature every 60 seconds for 300 seconds, we will make a table with time intervals and corresponding temperature readings.
Finally, we can use the formula to find the final temperature of the metal and water. Let's assume that the mass of water and metal are equal, and both are 100 grams. The specific heat capacity of water is 4.18 J/g °C, while the specific heat capacity of the metal is 0.91 J/g °C.
Heat lost by metal = Heat gained by waterm * c * ΔT(metal) = m * c * ΔT(water)100 * 0.91 * (50 - T) = 100 * 4.18 * (T - 20) 4550 - 91T = 418T - 8364.37T = 83.64T = 1.88°C
Therefore, the final temperature of the metal is 1.88°C, while the final temperature of water is 50°C. The final temperature of both is 25.94°C (average of 1.88°C and 50°C).
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
To determine the concentration of SO4 2– ion in a sample of groundwater, 100.0 mL of the sample is titrated with 0.0250 M Ba(NO3)2, forming insoluble BaSO4. If 7.48 mL of the Ba(NO3)2 solution is required to reach the end point of the titration, what is the molarity of the SO4 2–?
Answers
Answer:
1.87x10⁻³ M SO₄²⁻
Explanation:
The reaction of SO₄²⁻ with Ba²⁺ (From Ba(NO₃)₂) is:
SO₄²⁻(aq) + Ba²⁺(aq) → BaSO₄(s)
Where 1 mole of SO₄²⁻ reacts per mole of Ba²⁺
To reach the end point in this titration, we need to add the same moles of Ba²⁺ that the moles that are of SO₄²⁻.
Thus, to find molarity of SO₄²⁻ we need to find first the moles of Ba²⁺ added (That will be the same of SO₄²⁻). And as the volume of the initial sample was 100mL we can find molarity (As ratio of moles of SO₄²⁻ per liter of solution).
Moles Ba²⁺:
7.48mL = 7.48x10⁻³L ₓ (0.0250moles / L) = 1.87x10⁻⁴ moles of Ba²⁺ = Moles of SO₄²⁻
Molarity SO₄²⁻:
As there are 1.87x10⁻⁴ moles of SO₄²⁻ in 100mL = 0.1L, molarity is:
1.87x10⁻⁴ moles of SO₄²⁻ / 0.1L =
1.87x10⁻³ M SO₄²⁻Calculate the amount of heat energy required to melt 550.0 grams of copper, already at its melting point. NEED ASAP
Answers
Answer:
18,300 J
Explanation:
In order to be able to answer this question, you must know the value of water's enthalpy of fusion,
, which is listed as
ΔH↔ = 18,300J
Now, a substance's enthalpy of fusion tells you how much heat is needed in order to convert of said substance from solid at its melting point to liquid at its melting point.
In water's case, an enthalpy of fusion equal to
333.55 J g
333.55 J
of heat.
Your ice cube has a mass of
55.0 g
What is a reaction rate?
Answers
Answer:
A reaction is the time that is required for a chemical reaction to go essential to completion
Balance the equations by putting the necessary coefficients in the blanks. Normally we do not write 1s when balancing, but for this particular question you need to include them for full credit. __Na3N___ Na +__ N2 ___H3PO4 + __ KOH __K3PO4 + __ H2O __ N2 +__ H2 __ NH3 __H2O2 __ O2 + __ H2O __ Zn + __ HCl __ ZnCl2 + __H2 __ C2H6 + __ O2 __ CO2 + __H2O __ CuCl2 + __H2S __ CuS + __HCl

Answers
Balancing a chemical equation is the process of ensuring that the number of atoms of each element in the reactants is equal to the number of atoms of that same element in the products.
Balance the chemical eqations given in the problem?
Na3N → 3 Na + ½ N2H3PO4 + 3 KOH → K3PO4 + 3 H2ON2 + 3 H2 → 2 NH3H2O2 → O2 + 2 H2OZn + 2 HCl → ZnCl2 + H2C2H6 + 7/2 O2 → 2 CO2 + 3 H2OCuCl2 + H2S → CuS + 2 HClChemical equations are used to describe the reactants and products in a chemical reaction. These equations are written using chemical formulas and symbols, indicating the types and numbers of atoms or molecules involved in the reaction. However, these equations must be balanced to obey the law of conservation of mass, which states that the total mass of the reactants must equal the total mass.
To learn more about chemical equation, visit: https://brainly.com/question/29886207
#SPJ1
Fun With Predicting Reaction Products
I erased my answers so far but i’m confused about every thing on this paper. Pleaseeeee help thanks

Answers
To predict the products of such a reaction, see what happens if the chemical breaks into smaller, familiar products such as water, carbon dioxide, or any of the gaseous elements
Describe the type of reaction that was indicated.AgNO3+Na2SO4→AgSO4+2NaNO3 is an exchange reaction.
Na + O2→ Na2O .
is an exchange reaction.
Thus, we refer to these processes as redox. Na is oxidized by losing electrons in reaction (a), while O is reduced by gaining electrons, forming O2-ions.
A single-displacement reaction would be Mg + HBr > MgBr2 + H2 HBr + Mg. In this process, magnesium creates magnesium bromide by swapping out hydrogen from HBr (MgBr2).
The decomposition process CuSO4(aq) + Zn(s) ZnSO4(aq) + Cu(s) is an illustration.
To learn more about reaction refers to:
https://brainly.com/question/11231920
#SPJ1
The reactant concentration in a zero-order reaction was 8.00×10−2 M
after 140 s and 4.00×10−2 M after 400 s
. What is the rate constant for this reaction?
Answers
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1, depending on which rate was used to calculate it.
Determining the rate constantThe rate of the reaction is given by the equation:
Rate = -k[A]
where k is the rate constant and [A] is the concentration of the reactant.
Rate at t=140 s:
Rate = (8.00×10−2 M - 0 M) / (140 s - 0 s)
= 5.71×10−4 M/s
Rate at t=400 s:
Rate = (4.00×10−2 M - 0 M) / (400 s - 0 s)
= 1.00×10−4 M/s
Since this is a zero-order reaction, the rate of the reaction is constant, and we can use either rate to calculate the rate constant:
k = Rate / [A]
Using the rate at t=140 s:
k = 5.71×10−4 M/s / 8.00×10−2 M = 7.14×10−3 s−1
Using the rate at t=400 s:
k = 1.00×10−4 M/s / 4.00×10−2 M
= 2.50×10−3 s−1
The rate constant for the reaction is either 7.14×10−3 s−1 or 2.50×10−3 s−1.
Learn more on zero-order reaction https://brainly.com/question/21663229
#SPJ1
The largest contributed to water pollution is
Answers
Answer:
this si from google hope it helps
Explanation:
The Main Causes of Water Pollution in the U.S.
Runoff from Agricultural Operations. Agriculture represents one of the biggest sources of water pollution in the country. ...
Runoff and Nonpoint Source Pollution. ...
Industrial Activities. ...
Leakage from Underground Storage and Piping. ...
Leaking Sewers. ...
Vehicle Emissions. ...
Landfill Leakage. ...
Hazardous Waste.
Answer:
Runoff and Non-point Source Pollution.
Explanation:
it's caused by rainfall or snow-melt moving over and through the ground. As the runoff moves, it picks up and carries away natural and human-made pollutants, finally depositing them into lakes, rivers, wetlands, coastal waters and ground and NPS is it's abbreviation.
5. The gases in a hair spray can are at a temperature of 37 °cand a pressure of 5.6 atm. If the gases in the can reach apressure of 4.4 atm, the can will explode. To what temperaturemust the gases be raised in order for the can to explode? (Donot try this at home)
Answers
Answer
243.69 K
Explanation
Given:
Initial temperature, T₁ = 37 °C = (37 + 273.15 K) = 310.15 K
Initial pressure, P₁ = 5.6 atm
Final pressure, P₂ = 4.4 atm
What to find:
The temperature to which the gases must be raised in order for the can to explode.
Step-by-step solution:
According to Amonton's Law, it states that the pressure of an ideal gas varies directly with the absolute temperature when the volume of the sample is held constant.
The final temperature of the gas can be calculated using Amonton's law formula given by
\(\frac{P_1}{T_1}=\frac{P_2}{T_2}\)Putting the values of the given parameters into the equation:
\(\begin{gathered} \frac{5.6atm}{310.15K}=\frac{4.4atm}{T_2} \\ \\ T_2=\frac{4.4atm\times310.15K}{5.6atm} \\ \\ T_2=243.69\text{ }K \end{gathered}\)The temperature to which the gases must be raised in order for the can to explode is 243.69 K
How many grams of hydrogen are present in 10.05 grams of water?
Answers
In water (H2O), there are two atoms of hydrogen (H) and one atom of oxygen (O) per molecule. The atomic mass of hydrogen is approximately 1.008 g/mol and the molecular mass of water is approximately 18.015 g/mol.
To calculate the grams of hydrogen in 10.05 grams of water, we need to first determine the number of moles of water present:
moles of water = mass of water / molecular mass of water
moles of water = 10.05 g / 18.015 g/mol
moles of water = 0.5575 mol
Since there are two hydrogen atoms in each molecule of water, the number of moles of hydrogen is twice the number of moles of water:
moles of hydrogen = 2 x moles of water
moles of hydrogen = 2 x 0.5575 mol
moles of hydrogen = 1.115 mol
Finally, we can calculate the grams of hydrogen by multiplying the number of moles of hydrogen by the atomic mass of hydrogen:
grams of hydrogen = moles of hydrogen x atomic mass of hydrogen
grams of hydrogen = 1.115 mol x 1.008 g/mol
grams of hydrogen = 1.12352 g
Therefore, there are approximately 1.12352 grams of hydrogen present in 10.05 grams of water.
What is hydrogen?Hydrogen is a chemical element with the symbol H and atomic number 1. It is the lightest and most abundant element in the universe, constituting roughly 75% of all baryonic mass. Hydrogen is a colorless, odorless, and tasteless gas that is highly flammable.
Learn about hydrogen here https://brainly.com/question/24433860
#SPJ1
if the body is moving with uniform acceleration then, eng of motion are given as s = u+v/2+t
Answers
Yes, s = u+v/2+t, where s is the displacement, u is the beginning velocity, v is the end velocity, and t is the time required, is the equation of motion for a body travelling with uniform acceleration.
The basic law of motion, which states that a body's rate of change in displacement is directly proportional to its velocity, provides the basis for this equation. The equation of motion for a body travelling with constant acceleration, s = ut + 1/2at2, may be used to derive it.
The equation of motion for a body travelling with uniform acceleration is given by replacing the value of an as (v-u)/t and getting s = u+v/2+t. This formula is only accurate when the body's acceleration is constant and uniform.
Learn more about acceleration at:
https://brainly.com/question/12550364
#SPJ1
What would be the volume in liters of an 25.15 liter sample of gas at 201 °C and 2.31 atm if conditions were changed to STP?
Answers
The volume of the gas at STP would be 23.93 liters.
The volume of gas at STP (Standard Temperature and Pressure), we need to use the Ideal Gas Law, which states that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is temperature. First, we need to calculate the number of moles of gas in the initial sample. We can use the formula n = PV/RT, where P is the initial pressure, V is the initial volume, R is the gas constant, and T is the initial temperature.
n = (2.31 atm) x (25.15 L) / [(0.0821 L atm/mol K) x (201 + 273.15 K)]
n = 1.067 moles
Now, we can use the molar volume of gas at STP, which is 22.4 L/mol, to calculate the volume of gas at STP.
V = n x 22.4 L/mol
V = 1.067 moles x 22.4 L/mol
V = 23.93 L
Therefore, the volume of the gas at STP would be 23.93 liters.
For more such questions on gas
https://brainly.com/question/25736513
#SPJ11
Step 1: She pours a colorless solution into a beaker that contains another colorless liquid. Yellow particles appear in the liquid.
Step 2: She lets the yellow particles settle in the beaker and then separates the colorless liquid by decantation.
What has most likely occurred in the beaker?
a chemical change in step 1 and a physical change in step 2
a physical change in step 1 and a chemical change in step 2
chemical changes in both step 1 and step 2
physical changes in both step 1 and step 2
Answers
It is most likely Option A: that a chemical change occurred in step 1 and a physical change occurred in step 2.
What is the chemical change?In step 1, the yellow particles appeared in the liquid, indicating a chemical change. This could be a result of a chemical reaction between the two colorless liquids, or the addition of a reagent that caused a reaction. This change is characterized by the formation of new substances, which are the yellow particles.
In step 2, the colorless liquid was separated from the yellow particles by decantation. Decantation is a physical process that separates immiscible liquids or solid particles from a liquid by allowing them to settle and then carefully pouring off the liquid.
Therefore, This change is characterized by the separation of components of a mixture, no new substances are formed.
Learn more about beaker from
https://brainly.com/question/30191198
#SPJ1
What is one way humans can preserve biodiversity
Answers
Answer:
Reducing the amount of water you use, by having a 5-minute shower or not running the water when washing up the dishes, can help protect vital wetlands. Plant scientists are also working to help conserve by developing crop varieties that use less water.
Explanation:
Answer:
1. Most of the endangered organisms may represent a source of income.
2. The conservation or preservation may conflict with morals.
3. The role of the species or organisms might not be understood.
Explanation:
Biodiversity simply means the amount or number of living organisms that exist in the world.
The practice of protecting these existing living organisms for specific known purposes is regarded as the preservation or conservation of biodiversity.
This process or practice of preserving biodiversity is important to meet most of the human needs, such as generating income, source of food and fuel among others.
PLEASE HELPPP ME
Use the following balanced chemcial equation to answer the question below.
CaBr2 + Na2SO4 --> CaSO4 + 2NaBr
1. How many moles of sodium bromide can be made from 5 moles of calcium bromide
2. How many grams of calcium sulfate will be made from 2.00 moles of calcium bromide
3. How many grams of calcium bromide will be needed to make 845 grams of calcium sulfate
Answers
Answer:
1) (5 moles of calcium bromide) x 2 / 1 = 10 moles
2) (2.00 moles of calcium bromide) = 2.00 moles of calcium sulfate
so (2.00) x (136.1 g/mol) = 272.2 g
3) (845 grams of calcium sulfate) / 136.14 g/mol = 6.21 moles
6.21 moles of calcium sulfate = 6.21 moles of calcium bromide
(6.21 moles of calcium bromide) x 199.89 g/mol = 1241.32 g
A chemist reacts 10.5 g of Ag with 7.5 g of s in the reaction from the previous question what is the percent yield of a chemist actually obtains 9.8 g of ag2s
Answers
If 10.5 g of Ag and 7.5 g of S are reacted and 9.8 g of \(Ag_2S\) is obtained, the percent yield of the reaction would be 81.4%
Percent yieldAg and S react to form \(Ag_2S\) as follows:
\(2Ag + S -- > Ag_2S\)
The mole ratio of Ag to S is 2:1.
10.5 g of Ag = 10.5/108
= 0.0972 moles
7.5 g of S = 7.5/32
= 0.2344 moles
In other words, Ag is limiting in availability.
The mole ratio of Ag to \(Ag_2S\) = 2:1
0.0972 Ag is equivalent to 0.0972/2 = 0.0486 moles of \(Ag_2S\)
0.0486 moles \(Ag_2S\) weighs = 0.0486 x 247.8
= 12.04 grams
percent yield of \(Ag_2S\) = 9.8/12.04 x 100%
= 81.4%
The percent yield of \(Ag_2S\) that the chemist actually obtained is 81.4%.
More on percent yield can be found here: https://brainly.com/question/17042787
#SPJ1
The metric system is only used in France? True or false
Answers
Answer:
false
Explanation:
The metric system is used in all but 3 countries in the world
Please i meed help quick and thank you

Answers
It is the 4th scenario is the dependent event. There are 7 gold tokens and 4 silver tokens in a cup. The first student randomly draws a gold token and keeps it. A second student randomly draws a gold token from the cup.
How did we identify the dependent event?The fouth scenario is a dependent event because the probability of the second student drawing a gold token is affected by the outcome of the first student's draw.
If the first student draws a gold token, then there are only 6 gold tokens left in the cup, the probability changes. but if the first student does not draw a gold token, then there are 7 gold tokens left in the cup, the probability will remain the same
Find more exercises on dependent events;
https://brainly.com/question/11473170
#SPJ1
which of the following is true about a chemical reaction in which the reactant stores less energy than the product
Answers
The reactant in a chemical process that releases less energy than the result does the following. Heat absorption will help the reaction move forward.
Heat absorption refers to the process by which a material or object takes in and retains thermal energy from its surroundings. This can occur through several mechanisms, including conduction, convection, and radiation.
The ability of a material to absorb heat is determined by its specific heat capacity, which is a measure of how much energy is required to raise the temperature of the material by a given amount. Materials with a high specific heat capacity can absorb more heat without experiencing a significant increase in temperature.
Heat absorption has important implications in many areas of science and engineering, including thermodynamics, materials science, and climate science. It is also relevant to everyday life, as it affects the performance of various devices and systems, such as air conditioning units, solar panels, and cooking appliances.
Learn more about Heat absorption here:
https://brainly.com/question/13179315
#SPJ4
The complete question is:
Which of the following is true about a chemical reaction in which the reactant stores less energy than the product?
During the titration of a diluted vinegar sample with a sodium hydroxide solution, the volume of sodium hydroxide used was less than expected. Which of the following could account for the lower than expected volume?
A. The sodium hydroxide solution had been allowed to stand exposed to the air for a long time prior to the titration.
B. The volumetric flask used to prepare the diluted vinegar solution was rinsed with water prior to use.
C. The burette used to deliver the sodium hydroxide solution was rinsed with water prior to use.
D. The pipette used to deliver the vinegar solution was rinsed with water prior to use.
Answers
Answer:
B and D could be true
Explanation:
A volume of sodium hydroxide less than expected could occurs for two reasons:
The real concentration of sodium hydroxide was higher than expected or the amount of vinegar added was less than expected:
A. The sodium hydroxide solution had been allowed to stand exposed to the air for a long time prior to the titration. FALSE. A long expose to the air decreases concentration of the NaOH.
B. The volumetric flask used to prepare the diluted vinegar solution was rinsed with water prior to use. TRUE. You add a less amount of vinegar doing you require less amount of NaOH than expected.
C. The burette used to deliver the sodium hydroxide solution was rinsed with water prior to use. FALSE. Thus, you add a less amount of NaOH than expected. To explain the matter, you add more NaOH than expected.
D. The pipette used to deliver the vinegar solution was rinsed with water prior to use. TRUE. Again, you are adding a less amount of Vinegar than expected doing the necessary NaOH during titration less than expected
The preferred conformation or Cis -3-tert - buty1-1- methyl cyclohexane is the one in which: _________.
A) the tert-butyl group is axial and the methyl group is equatorial.
B) the methyl group is axial and the tert-butyl group is equatorial.
C) both groups are axial.
D) both groups are equatorial.
E) the molecule exists in a boat conformation.
Answers
Answer:
D) both groups are equatorial.
Explanation:
To solve this question we can start by drawing the molecule in using the structure of the hexagon. In this structure, we have the methyl group on carbon 1 and the terbutyl group on carbon 3, additionally, if we have a cis structure both groups must have the same type of bond (in this case the wedge bond). (See figure 1)
When we write the chair structure, we must keep the same structure. That is, methyl must be on carbon 1 and terbutyl on carbon 3. Also, the cis configuration must be maintained. With this in mind, we can choose the equatorial configuration for methyl on carbon 1 (since the position equatorial is the one that has less steric impediment and more stability). If this is true for carbon 1, we must place the terbutyl group on carbon 3 in the same configuration (i.e. cis). Therefore, on carbon 3 we must place the tert-butyl down on carbon 3, that is, in the equatorial position. (See figure 1).
Therefore, in the chair-like structure, both groups must be in an equatorial position.
I hope it helps!

What is true of all matter?
A. It pushes or pulls on objects.
B. You can see it.
C. It gives off heat energy.
D. It has mass.
Answers
HELP ME OUT PLEASE!!
Barium sulfate, BaSO4 is a white crystalline solid that is insoluble in water. It is used by doctors to diagnose problems with the digestive system,
Barium hydroxide, Ba(OH)2 is also a white crystalline solid and is used in wastewater treatment.
How many more oxygen atoms are represented in the formula for barium sulfate than in the formula for barium hydroxide?
A) 2
B) 4
C) 1
D) 8
Answers
Answer: 2
Explanation:
Ba(OH)2 contains two oxygen atoms
BaSO4 contains four oxygen atoms.
This means that barium sulfate contains two more oxygen atoms than barium hydroxide in its formula.
Hope this helps!! :)
According to formula BaSO₄, Barium sulfate contains 2 more oxygen atom.
The correct option is (A) 2
Barium sulfate's distinguishing featureBa(OH)₂ has two oxygen atoms in it.
Four oxygen atoms may be found in BaSO₄.
This indicates that the formula of barium sulfate has two extra oxygen atoms than the formula of barium hydroxide.
Barium sulfate, often known as BaSO4, is a transparent white pigment that has a long history. It is produced by combining sulfuric acid with barium hydroxide and other sources of barium.
Chemically speaking, barium hydroxide has the formula Ba(OH)2. The monohydrate of barium, sometimes referred to as baryta or baryta-water, is one of the element's main compounds. The typical commercial form of monohydrate is this white granular substance.
Learn more about the Barium sulfate with the help of the given link:
https://brainly.com/question/11334621
#SPJ2
What is the mass of 6.02 x 1024 molecules of the compound HCl?
Answers
Answer:
First, we need to determine the molar mass of HCl.
The molar mass of HCl = the mass of hydrogen (1.008 g/mol) + the mass of chlorine (35.45 g/mol) = 36.45 g/mol.
Next, we can use Avogadro's number (6.02 x 10^23 molecules/mol) to convert the number of molecules to moles:
6.02 x 10^24 molecules / 6.02 x 10^23 molecules/mol = 10 moles
Finally, we can use the molar mass to convert moles to grams:
10 moles x 36.45 g/mol = 364.5 grams
Therefore, the mass of 6.02 x 10^24 molecules of HCl is 364.5 grams.
:. It means 1 mole of Hcl
:. To find the mass of HcL
no of moles = mass/ molar mass
To get the molar mass of HCL {H=1 CL=35.5}
:. H+CL = 1+ 35.5 =36.5
So we have our molar mass and number of moles now
Then we input it in the eqn
1=x/36.5
X= 36.5g of HCl
The nucleus of an atom contains which subatomic partcles?
Answers
Answer:
The nucleus contains two types of subatomic particles, protons and neutrons. The protons have a positive electrical charge and the neutrons have no electrical charge. A third type of subatomic particle, electrons, move around the nucleus. The electrons have a negative electrical charge.
Will give brainliest for someone to help me with this
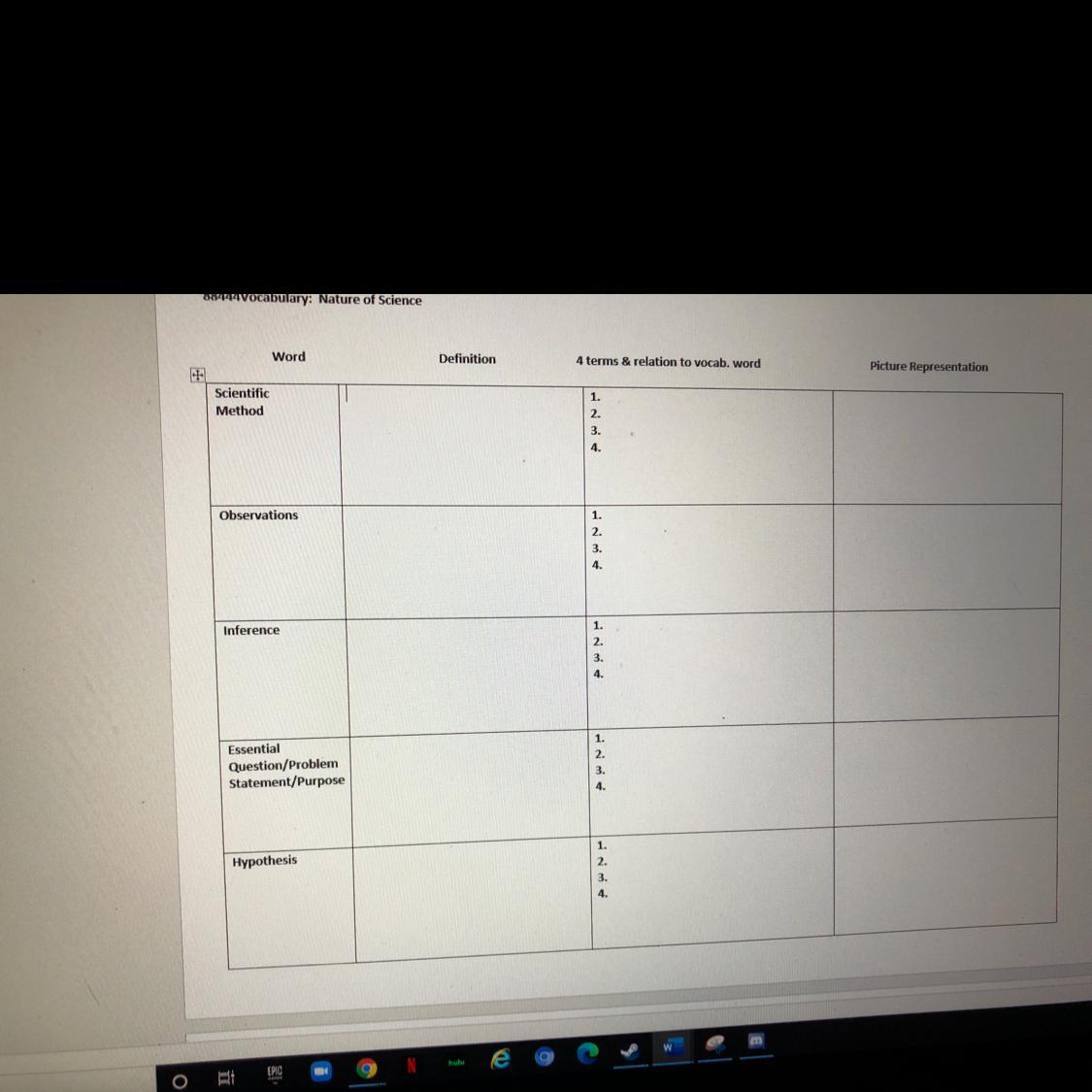
Answers
Answer:
Box #1: a method of procedure that has characterized natural science since the 17th century, consisting in systematic observation, measurement, and experiment, and the formulation, testing, and modification of hypotheses.
Explanation:
The terms: The scientific method describes the processes by which scientists gain knowledge about the world. It's characterized by six key elements: questions, hypotheses, experiments, observations, analyses, and conclusions. These elements are interrelated steps, so they don't always function in the same order.
The Relation: When conducting research, scientists use the scientific method to collect measurable, empirical evidence in an experiment related to a hypothesis (often in the form of an if/then statement), the results aiming to support or contradict a theory.
The arrows in the chart below represent phase transitions.
Solid
Liquid
16
Gas
Which arrows represent the phase transitions in which heat energy is gained?
1, 2, and 3
2,3, and 5
3, 4, and 5
4,5, and 6
Answers
Answer:
A: 1, 2, and 3
Explanation:
mY ANSWER GOT DELETED ITS WHAT I SAID BEFORE ITS ON EDGE2020
Answer:
A. 1, 2, and 3
Explanation:
Correct on Edge 2022!!!
Good luck everyone, you got this! Have a great day!
HELP PLEASE PLEASE PLEASE. Can anyone tell me how to separate the following mixture
A) ethanol in water
B) boiling the mixture of chloride crystals with water
C) pure water from muddy water
D) sodium chloride in water
E) sodium carbonate in water
F) chlorophyll from leaves
G) mixture of acetic acid and alcohol
H) serum from blood sample
I) kerosene from water
J) ammonium chloride in sand
I NEED CORRECT ANSWERS ONLY.
HURRY UP PLEASE. I WILL MARK AS BRAINLIEST
Answers
A) Ethanol in water: Distillation.
B) Boiling the mixture of chloride crystals with water: Evaporation.
C) Pure water from muddy water: Filtration.
D) Sodium chloride in water: Evaporation or Crystallization.
E) Sodium carbonate in water: Filtration or Evaporation.
F) Chlorophyll from leaves: Extraction using a suitable solvent like ethanol.
G) Mixture of acetic acid and alcohol: Distillation.
H) Serum from blood sample: Centrifugation.
I) Kerosene from water: Separatory funnel or Decantation.
J) Ammonium chloride in sand: Sublimation or Dissolving in water and Filtration.
A) Ethanol in water: Distillation can be used to separate ethanol from water based on their different boiling points.
B) Boiling the mixture of chloride crystals with water: By heating the mixture, the water will evaporate, leaving behind the chloride crystals.
C) Pure water from muddy water: Filtration can be used to separate the solid particles (mud) from the water.
D) Sodium chloride in water: Evaporation can be used to separate sodium chloride from water by heating the mixture until the water evaporates, leaving behind the salt.
E) Sodium carbonate in water: Filtration can be used to separate solid sodium carbonate from water, similar to muddy water.
F) Chlorophyll from leaves: Extraction using a suitable solvent like ethanol or acetone can be used to separate chlorophyll from leaves.
G) Mixture of acetic acid and alcohol: Distillation can be used to separate the mixture based on their different boiling points.
H) Serum from blood sample: Centrifugation can be used to separate the serum, which is the liquid part of blood, from the solid components like cells.
I) Kerosene from water: Separatory funnel or decantation can be used to separate the immiscible liquids by pouring off the top layer (kerosene) from the bottom layer (water).
J) Ammonium chloride in sand: Sublimation can be used to separate ammonium chloride by heating the mixture, causing the ammonium chloride to vaporize and then condense back into solid form in a cooler region, leaving the sand behind.
Know more about Sublimation here:
https://brainly.com/question/16789108
#SPJ8
Nitrogen has a larger atomic radius than oxygen true or false
Answers
Answer:
true. i looked it up and it says nitrogen is larger.