Motor trend magazine reported that 28.6% of all cars are red. mario is standing by
the side of the road watching cars go by. what is the probability that at least 4 out
of the next 8 cars that go by are red?
Answers
To solve this problem, we can use the binomial distribution formula. The formula is: P(X>=k) = 1 - Σ (i=0)^(k-1) [(n choose i) * p^i * (1-p)^(n-i)]
Where X is the number of red cars in the next 8 cars, k is the number of red cars we want to find (at least 4), n is the total number of cars (8), and p is the probability of a car being red (0.286).
So, the probability of getting at least 4 red cars out of the next 8 cars can be calculated as:
P(X>=4) = 1 - Σ (i=0)^(3) [(8 choose i) * (0.286)^i * (1-0.286)^(8-i)]
P(X>=4) = 1 - [ (8 choose 0) * (0.286)^0 * (0.714)^8 + (8 choose 1) * (0.286)^1 * (0.714)^7 + (8 choose 2) * (0.286)^2 * (0.714)^6 + (8 choose 3) * (0.286)^3 * (0.714)^5 ]
P(X>=4) = 1 - [ 1 * 1 * 0.335 + 8 * 0.286 * 0.408 + 28 * 0.081 * 0.492 + 56 * 0.023 * 0.570 ]
P(X>=4) = 1 - 0.643
P(X>=4) = 0.357
Therefore, the probability that at least 4 out of the next 8 cars that go by are red is 0.357 or 35.7%.
To know more about binomial distribution visit:-
https://brainly.com/question/29163389
#SPJ11
Related Questions
Complete the sentences to explain your choice. Match the words in the left column to the appropriate blanks in the sentences on the right. When comparing HF and HCl, HCl is a stronger acid because the bond is ___When comparing H2O and HF, HF is a stronger acid because the bond is ___When comparing H2Se and H2S, H2Se is a stronger acid because the bond is ___less polar more polarweakerstronger
Answers
Answer:
HCl is a stronger acid because the bond is weaker
Hf is a stronger acid because the bond is more polar
H2Se is a stronger acid because the bond is weaker.
Explanation:
Weaker and more polar acids make stronger acids.
a. When comparing HF and HCl, HCl is a stronger acid because the bond is stronger.
b. When comparing H2O and HF, HF is a stronger acid because the bond is weaker.
c. When comparing H2Se and H2S, H2Se is a stronger acid because the bond is weaker.
In comparing the acidity of acids, we can consider the strength of the bond between the hydrogen atom and the non-metallic atom in the acid. The weaker the bond, the more easily the hydrogen ion can be donated to a base, making the acid stronger.
a. This is because HCl has a weaker bond between hydrogen and chlorine, and it readily donates hydrogen ions to a base.
b. This is because HF has a stronger bond between hydrogen and fluorine, which makes it harder to donate hydrogen ions to a base.
c. This is because H2Se has a weaker bond between hydrogen and selenium, and it readily donates hydrogen ions to a base.
To know more about "Acids" refer here:
https://brainly.com/question/12814523#
#SPJ11
Which of the following is a technique for brainstorming new ideas by asking a lot of questions? Select one: a. starbursting b. spider-webbing c. firesparking d. star-brighting
Answers
The technique for brainstorming new ideas by asking a lot of questions is called starbursting. Option a.
This technique involves generating a lot of questions related to a particular topic and then answering each question with a set of new questions. By doing this, it helps to identify different aspects of the problem and to come up with new ideas that may not have been considered before.
This method encourages critical thinking and helps to explore different possibilities and perspectives. It is a useful tool for problem-solving, innovation, and decision-making. In summary, starbursting is a powerful technique that helps to unlock creativity and generate fresh ideas. Answer option a.
More on brainstorming: https://brainly.com/question/17382444
#SPJ11
Consider the following three-step mechanism for a reaction: Cl2 (g) ⇌ 2 Cl (g) Fast Cl (g) CHCl3 (g) → HCl (g) CCl3 (g) Slow Cl (g) CCl3 (g) → CCl4 (g) Fast Identify the intermediates in the mechanism.
Answers
The intermediates in the given three-step mechanism are Cl (g) and CCl3 (g).
In the mechanism, Cl2 (g) is in equilibrium with 2 Cl (g), indicating that Cl (g) is an intermediate formed during the reaction. This means that Cl2 (g) breaks apart into Cl (g) molecules, which then go on to react with other species in subsequent steps.
In the second step, Cl (g) reacts with CHCl3 (g) to form HCl (g) and CCl3 (g). Here, Cl (g) is consumed as it reacts with CHCl3 (g) to produce the products.
In the third step, Cl (g) reacts with CCl3 (g) to form CCl4 (g). This step consumes Cl (g) as it reacts with CCl3 (g) to produce the final product.
Overall, the intermediates in this three-step mechanism are Cl (g) and CCl3 (g). They are formed in intermediate steps of the reaction and are consumed in subsequent steps to yield the final products.
To learn more about equilibrium click here : brainly.com/question/30694482
#SPJ11
20. a solution containing an unknown metal ion is sprayed into an open flame, giving rise to an orange color by eye. upon researching metal ions which burn with this color, you find several candidate ions which also burn orange and are unsure how to identify the unknown. what test(s) would you perform to identify the identity of the unknown?
Answers
To identify the unknown metal ion that produces an orange color in a flame test, you could perform several tests like; Flame Coloration Test, Confirmatory Tests, Spectroscopic Analysis, pH and Solubility Tests, and Comparison with Known Samples.
Perform flame tests with known metal ions that are known to produce an orange color in a flame e.g., sodium, calcium, lithium, etc. Observe the color of the flame produced by the unknown metal ion and compare it to the reference flame colors.
Once you have narrowed down the possibilities based on the flame color, you could perform additional confirmatory tests to further identify the unknown metal ion.
Also, we use spectroscopic techniques, such as atomic absorption spectroscopy (AAS) or emission spectroscopy, to analyze the unknown solution and determine the concentration and identity of the metal ions present.
You could perform pH and solubility tests on the unknown solution to determine its acidity or basicity and observe any characteristic changes in color or precipitation.
If you have access to known samples of metal ions with similar flame colors, you could compare the unknown solution with these known samples using various tests, such as chemical reactions, spectroscopic analysis, or physical properties, to determine similarities or differences that may help in identifying the unknown metal ion.
To know more about flame tests here
https://brainly.com/question/6357832
#SPJ4
Which group of nonmetals will react with group 1 metals to form a compound in a one-to-one ratio?.
Answers
Alkaline earth metals will react in a 1:1 ratio with group 16 nonmetals to generate a compound.
Our two ratios would combine. As a result of their greater positive charge and smaller iniquity. Compared to alkali metals, alkaline earth metals have a significantly stronger tendency to form a complex with the Lewis base.
Alkali metals, which contain a single valence electron, and the halogens react aggressively. The two components come together to create salt. For instance, the reaction between the alkali metal sodium (Na) and the halogen chlorine (Cl) yields table salt, or sodium chloride (NaCl).
The group 1 element Li, Na, K, Rb, Cs, and Fr are alkali metals. When sliced, the alkali metals are all supple, metallic, and lustrous.
As an illustration, each member of Group 1 surrenders one electron to become a 1+ cation. A 1- anion can be created by adding one electron to any element in Group 17. Ionic compounds can be created when elements from Groups 1 and 17 mix one to one.
Learn more about non-metals here:
https://brainly.com/question/16749127
#SPJ4
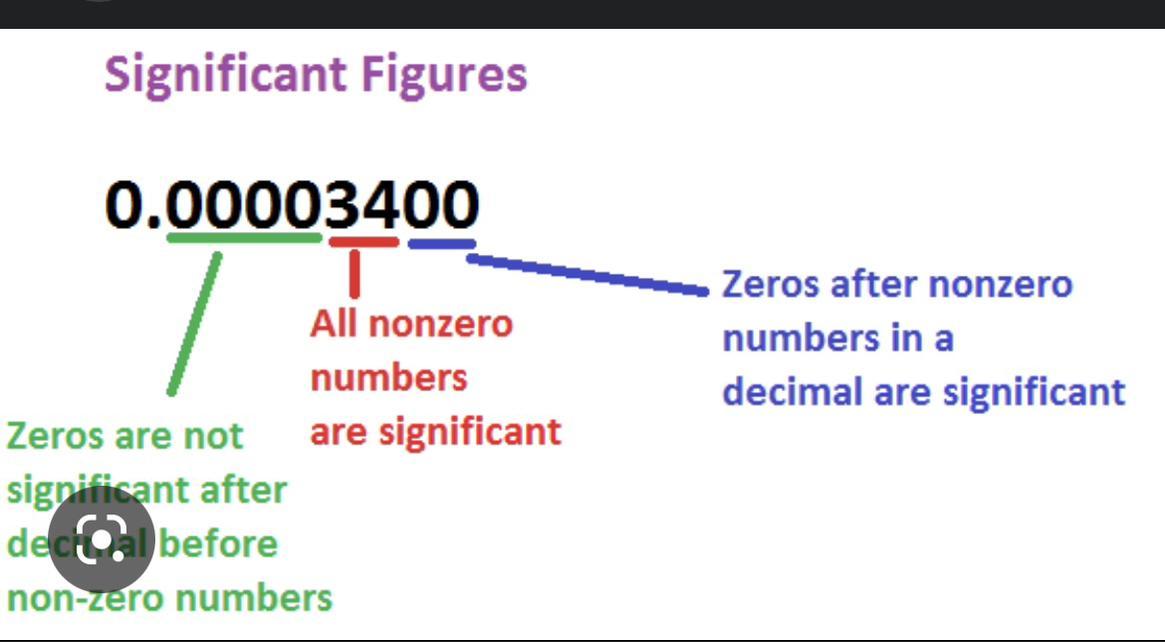
how would you round 34.9279 if there was 3 sig figs and 4 sig figs
Answers
Name the most abundant isotope for these elements: Na = ________
P = _______ Mn = ________
Answers
Answer: 11, Na, 23, 100, −9.529 ... phosphorus, 15, P, 31, 100, −24.441 ... manganese, 25, Mn, 55, 100, −57.706.
Explanation: Make me Brainelist
what is true about a polar covalent bond? select the correct answer below: they are characterized by a partial positive charge on one atom and a partial negative charge on the other. the electrons are shared equally between the atoms in the bond. the electrons are transferred from one atom to the other in the bond. the electrons are absorbed into the nucleus of one atom in the bond.
Answers
Polar covalent bonds are characterized by a partial positive charge on one atom and a partial negative charge on the other. Option a is correct.
In a polar covalent bond, the electrons are not shared equally between the atoms in the bond. Instead, one atom attracts the shared electrons more strongly than the other, resulting in a partial negative charge on the more electronegative atom and a partial positive charge on the less electronegative atom.
This creates a separation of charges across the bond, making it polar. The magnitude of the charge separation depends on the difference in electronegativity between the atoms. Hence option a is correct.
To know more about covalent bond, here
brainly.com/question/13915235
#SPJ4
mass measurements of the crucible, lid, and sample are performed only at room temperature. why is this technique necessary for a gravimetric analysis?
Answers
Mass measurements of the crucible, lid, and sample are performed only at room temperature because the heat from the crucible warms the surrounding air which rises then that air cools down and falls.
The process is necessary for gravimetric analysis as scales are calibrated at room temperature.
The heating is done so as the surrounding air is heated by the process of convection this will lead to inaccurate and unsteady readings which are rising and falling also heating is done in the crucible before heating is done so as to remove the air moisture.
Gravimetric analysis is a method of quantitative chemical analysis in which the constituent sought is converted into a substance that can separate from the sample and weighed.
Gravimetric analysis is dependent on accuracy so calibration is necessary as it maintains accuracy standardization and repeatability in measurements.
To learn more about gravimetric analysis
https://brainly.com/question/1528671?referrer=searchResults
#SJP4
at 320k and 16 atm pressure, the molar volume of ammonia, nh3, is about 10% less than the molar volume of an ideal gas. the best explanation for the actual volume being this much smaller than the ideal volume is that
Answers
At 320 K and 16 atm pressure, the molar volume of ammonia (NH3) is approximately 10% less than the molar volume of an ideal gas due to intermolecular forces and deviations from ideal behavior.
The molar volume of an ideal gas is based on the ideal gas law, which assumes that gas molecules do not interact with each other and occupy a negligible amount of space. However, real gases, including ammonia (NH3), deviate from this ideal behavior due to intermolecular forces and the finite size of gas molecules.
At 320 K and 16 atm pressure, the ammonia molecules are relatively close together, and intermolecular forces become significant. Ammonia molecules exhibit dipole-dipole interactions, where the positive end of one molecule attracts the negative end of another, leading to attractive forces between the molecules. These intermolecular forces reduce the available space for the gas molecules to move freely, causing the actual volume to be smaller than that predicted by the ideal gas law.
Additionally, the finite size of gas molecules also contributes to the deviation from ideal behavior. In reality, gas molecules have a certain size and occupy a small but non-negligible amount of space. At high pressures, such as 16 atm, the volume occupied by the ammonia molecules becomes more significant, further reducing the molar volume compared to an ideal gas.
Overall, the combination of intermolecular forces and molecular size effects leads to the molar volume of ammonia being approximately 10% less than the molar volume predicted by the ideal gas law at 320 K and 16 atm pressure.
To learn more about ideal gas click here:
brainly.com/question/30236490
#SPJ11
15. What will be the volume of a given mass of oxygen at 250C if it occupies 100cm3 at 150C.? (Pressure remain constant)
Answers
Answer:
V₂ = 167 cm₃
Explanation:
Because you are only dealing with volume and temperature, you can use Charles' Law to find the missing value. This formula looks like this:
V₁ / T₁ = V₂ / T₂
In this formula, "V₁" and "T₁" represent the initial volume and temperature. "V₂" and "T₂" represent the final volume and temperature. You have been given values for all of the variables except for final volume. Therefore, by plugging these values into the formula, you can simplify to find your answer.
V₁ = 100 cm³ T₁ = 150 °C
V₂ = ? T₂ = 250 °C
V₁ / T₁ = V₂ / T₂ <---- Charles' Law formula
(100 cm³) / (150 °C) = V₂ / (250 °C) <---- Insert values
(0.667) = V₂ / (250 °C) <---- Simplify left side
(0.667) x (250 °C) = V₂ <---- Rearrange
167 = V₂ <---- Simplify left side
What is represented by the dashed blue line and the solid black line in the image below? motion,vectors,scalars,distance,displacement

Answers
Answer:
Distance
Explanation:
There as you see the lines are in a following of a path, like marathon. IT also states above each structure. Start and finish, why would it show motion. It shows distance.
PLEASEEE HELPPP I SUCK AT CHEM :(
a 0.100M H2SO4 solution is neutralised with 10.00ml of a solution of 0.300M KOH
a) write a balanced equation for this reaction
b) what volume of sulfuric acid was neutralised
Answers
Answer:
a) H2SO4 + 2KOH -> 2H2O + K2SO4
b) 9.809 ml
Explanation:
Number of Moles = Mass/ Molar Mass
Therefore: Mass = Number of moles * Molar Mass
--------------------------------------------
Molar mass of H2SO4:
H2= 2.02
S= 32.07
O4= 64
--------------------------------------------
H2SO4 has the molar mass of 98.09
--------------------------------------------
the Moles of H2SO4 is given to be 0.100M
Therefore:
Mass= 98.09*0.1
= 9.809g
---------------------------------------------------
Assuming that 1 g= 1 ml, the volume of sulfuric acid is 9.809 ml.
How can you show using Pauli's exclusion principle that p sub shell can have only 6 electrons?

Answers
where l = subshell value.
"l"values of subshell are.
s = 0.
p = 1.
d = 2.
f = 3.
So in p orbital we have 6 electrons.
predict the sign on the thermodynamic quantities listed below based on the scene representing a physical change in a piston-cylinder assembly.
Answers
Based on a scene showing a physical change in a piston-cylinder assembly, the thermodynamic numbers indicated below are 760 torr 760.
What elements make up the first rule of thermodynamics?The first law of thermodynamics states that the following equation describes how the change in internal energy relates to the heat exchanged by the system and the work performed on or by the system: U = Q + W, where Q represents the heat energy that the system exchanged.
What are the thermodynamic quantities' two components?Extensive and intensive thermodynamic quantities are traditionally separated into these two categories. While intensive quantities are independent of system size, extensive quantities grow linearly with system size.
To know more about thermodynamic visit:-
https://brainly.com/question/1368306
#SPJ4
The original source of energy for this pond area
is the
(1) fish
(3) water
(2) Sun
(4) duck
Answers
Answer:
the sun!!
Explanation:
Ponds get their energy from the sun. As with other ecosystems, plants are the primary producers. The chlorophyll in aquatic plants captures energy from the sun to convert carbon dioxide and water to organic compounds and oxygen through the process of photosynthesis.
if you supplied calories to a sample of ethyl ether, how many grams could you vaporize at its boiling point
Answers
if calories were supplied to a sample of ethyl ether, you could vaporize 0.0046 grams at its boiling point.
The amount of ethyl ether that can be vaporized at its boiling point if calories are given to it can be determined using the following equation.
Q = m × ΔHv
where Q is the energy needed to vaporize the sample, m is the mass of the sample, and ΔHv is the molar heat of vaporization.
The molar heat of vaporization for ethyl ether is 26.0 kJ/mol.
To find the amount of ethyl ether that can be vaporized, we will use the formula Q = mcΔT,
where c is the specific heat of ethyl ether, which is 1.72 J/g°C, and ΔT is the change in temperature between the initial and final states.
To find the quantity of ethyl ether vaporized, we'll use the following formula:
Q = mcΔT = mΔHv = (m)(1.72 J/g°C)(34.6°C) = (m)(26.0 kJ/mol)
where m is the mass of ethyl ether in grams.
Let's solve the equation for m as follows:
m = (Q/ΔHv)(1/1000)(1/1.72)(1/34.6)g = (Q/ΔHv)(0.0004)g
where g is the mass of ethyl ether in grams, and Q/ΔHv is in units of mol.
Using the numbers provided, we get:
m = (300/26.0)(0.0004)gm = 0.0046 g
Therefore, if calories were supplied to a sample of ethyl ether, you could vaporize 0.0046 grams at its boiling point.
To know more about ethyl ether refer here: https://brainly.com/question/14284330#
#SPJ11
complete combustion of 5.60 g of a hydrocarbon produced 17.3 g of co2 and 7.95 g of h2o. what is the empirical formula for the hydrocarbon? insert subscripts as necessary.
Answers
The empirical formula of the hydrocarbon is \(CH_2.\)
What is the empirical formula?
The empirical formula of a compound represents the simplest, most reduced ratio of elements present in the compound. It shows the relative number of atoms of each element in the compound, without indicating the actual molecular structure.
To determine the empirical formula of the hydrocarbon, we need to find the ratios of C and H atoms in the compound.
Calculate the moles of \(CO_2\) produced:
Molar mass of \(CO_2\) = 12.01 g/mol + 2(16.00 g/mol)
= 44.01 g/mol
Moles of \(CO_2\)=
\(\frac{mass &of &CO_2}{molar &mass& of& CO_2} \\= \frac{17.3 g}{44.01 g/mol}\\ = 0.393 mol CO_2\)
Calculate the moles of \(H_2O\) produced:
Molar mass of \(H_2O\) = 2(1.01 g/mol) + 16.00 g/mol
= 18.02 g/mol
Moles of \(H_2O\) =
\(\frac{mass& of &H_2O}{ molar &mass& of &H_2O}\\= \frac{7.95 g}{18.02 g/mol }\\= 0.441 mol H_2O\)
Determine the moles of carbon and hydrogen:
Moles of C =\(0.393 mol &CO_2 *\frac{1 mol C }{1 &mol &CO_2}\)
= 0.393 mol C
Moles of H = \(0.441 mol &H_2O *\frac{2 mol &H }{1 mol &H_2O}\)
= 0.882 mol H
Find the simplest whole number ratio of C to H:
Divide both moles of carbon and hydrogen by the smaller value (0.393 mol):
Moles of C = \(\frac{0.393 mol C}{0.393 mol}\) = 1 mol C
Moles of H = \(\frac{0.882 mol& H}{0.393 mol}\) = 2.24 mol H
Therefore,the empirical formula of the hydrocarbon is\(CH_2.\)
To learn more about the empirical formula from the given link
brainly.com/question/1603500
#SPJ4
What mss of sulfamic acid is required to make 250cm3 of a 0.150mol/dm3 solution? please help

Answers
Answer:
sorry i dint understand can u ask it in a simpler way
Explanation:
estimate the uncertainty in your measurement of the object's apparent shift. for example, do you think your recorded measurements could be off by ten degrees?
Answers
The uncertainty in the measurement of the object's apparent shift could potentially be as large as ten degrees.
It is important to note that the uncertainty in measurement can vary depending on several factors, such as the precision of the measurement instrument, the methodology used, and the skill of the observer. While it is difficult to provide an exact value for the uncertainty without specific details, a ten-degree difference serves as a reasonable estimation.
This implies that the recorded measurements of the object's apparent shift may deviate by up to ten degrees from the true value. To reduce uncertainty, it is advisable to use more precise instruments, employ rigorous measurement techniques, and consider multiple observations to account for any potential errors.
For more questions like Uncertainty click the link below:
https://brainly.com/question/15103386
#SPJ11
Bonded Atoms: 5
Lone Pairs: 1
Electron Domain: 6
Ideal Bond Angle?
Hybridization?
Polar or NonPolar?
Answers
The molecule has six electron domains, consisting of five bonded atoms and one lone pair. The ideal bond angle is 90 degrees. The hybridization of the central atom would be sp3d2. The molecule may be polar or nonpolar depending on the nature and orientation of the bonded atoms and lone pair.
With 5 bonded atoms and 1 lone pair, the electron domain of the molecule is 6.
The ideal bond angle can be predicted using the VSEPR theory, which states that the electron domains in a molecule will arrange themselves to be as far apart as possible to minimize repulsion.
For a molecule with six electron domains, the ideal bond angle is 90 degrees.
The hybridization of the central atom can be determined using the number of electron domains present. In this case, the central atom has six electron domains, which corresponds to sp3d2 hybridization.
Whether the molecule is polar or nonpolar depends on the geometry of the molecule and the polarity of its bonds. Without knowing the specific molecule in question, it is difficult to determine whether it is polar or nonpolar.
Click the below link, to learn more about Bond Angle and hybridisation:
https://brainly.com/question/30230127
#SPJ11
Which kind of worm has a closed circulatory system?
planarian
O
a fluke
O a pinworm
O an earthworm
Answers
Answer:
o an earthworm
Explanation:
Answer:
an earthworm
Explanation:
Element X has two naturally occurring isotopes. The isotope with mass 62.93 amu has a
relative abundance of 69.2%. The isotope with mass 64.93 amu has a relative
abundance of 30.8%. Calculate the average atomic mass of Element X. What is the
identity of Element X?
Answers
Explanation:
To solve this, you multiply the mass of each isotope by its relative abundance as a decimal (for this question, it would be 62.93 * .692 and 64.93 * .308), then you add the two results together. That gives you the average atomic mass. Then, you look at the periodic table to find which element the mass matches to. If there is none, check your math.
Propanoic acid fromula
Answers
Answer:
C₃H₆O₂
Explanation:
Propionic acid is a colorless liquid with a sharp rancid odor. Produces irritating vapor. (USCG, 1999)
What is the molarity of a 9.13 L soda that contains 13.83 of sugar?
Answers
Answer:
1.52 M
Explanation:
Molarity of a solution is calculated as follows:
Molarity = number of moles (n) ÷ volume (V)
Based on the information given in this question,
Volume of soda (V) = 9.13 L
number of moles = 13.83 mol
Molarity = 13.83 ÷ 9.13
Molarity = 1.52 M
.. When water boils, you can see bubbles rising to the surface of
the water. Of what are these bubbles made?
a. air
b. hydrogen and oxygen gas
c. oxygen gas
d. water vapor
e. carbon dioxide gas
Answers
Answer:
Explanation:
It's water vapor. There is enough heat present to get the water to boil but not enough to break it into its chemical components (oxygen and hydrogen), so the answer is D.
Which is an example of a polymer?
Answers
Answer:There are two types of polymers: synthetic and natural. Synthetic polymers are derived from petroleum oil, and made by scientists and engineers. Examples of synthetic polymers include nylon, polyethylene, polyester, Teflon, and epoxy. ... Examples of naturally occurring polymers are silk, wool, DNA, cellulose and proteins.
Explanation:
chemistry
oxidation number
Answers
Answer:
Oxidation number also known as Oxidation State, its the total number of electrons that an atom either gains or loses in order to form a chemical bond with another atom. Each atom that participates in an oxidation-reduction reaction ( q.v.) is assigned an oxidation number that reflects its ability to acquire, donate, or share electrons.
Explanation:
hope this helps
When a solution containing M(NO3)2 of an unknown metal M is electrolyzed, it takes 74.1 s for a current of 2.00 A to to plate out 0.0737 g of the metal. The metal is
Answers
The metal in the solution is most likely copper.
How to determine the metal?To determine the metal, we need to use Faraday's law of electrolysis, which states that the amount of metal deposited at the cathode is directly proportional to the amount of charge passed through the cell.
The formula for Faraday's law is:
mass = (current × time × atomic mass) / (valence × Faraday's constant)
where:
mass is the amount of metal deposited
current is the current passed through the cell
time is the time for which the current is passed
atomic mass is the atomic mass of the metal
valence is the valence of the metal
Faraday's constant is a constant equal to the charge on one mole of electrons, 96,485 C/mol.
We can rearrange the formula to solve for the atomic mass of the metal:
atomic mass = (mass × valence × Faraday's constant) / (current × time)
Plugging in the given values, we get:
atomic mass = (0.0737 g × 2 × 96,485 C/mol) / (2.00 A × 74.1 s)
atomic mass ≈ 63.55 g/mol
Looking up the atomic masses of elements, we find that the closest match is copper (Cu), which has an atomic mass of 63.55 g/mol. Therefore, the metal in the solution is most likely copper.
Learn more about Faraday's law of electrolysis
brainly.com/question/30343167
#SPJ11
The electronegativities of carbon and sulfur are almost the same. Both elements form covalently bonded compounds with hydrogen. Why is hydrogen sulfide a polar compound while methane is a nonpolar compound?
a.Sulfur has a stronger attraction for electrons than does carbon
b.A hydrogen sulfide molecule has lone pairs of electrons
c.Sulfur forms ionic bonds with hydrogen while carbon forms covalent bonds
d.Sulfur ions are larger than carbon ions
Answers
Answer:
b. A hydrogen sulfide molecule has lone pairs of electrons
Explanation:
The presence of lone pairs indictates that the molecule will be polar unless its molecular geometry is linear or square planar. In the case of H2S, its shape is bent due to the lone pair and it is a slightly polar molecule.