Question 4 of 5
Which characteristic describes the living things in the domain Eukarya?
A. They can be made up of prokaryotic cells.
B. They are made up of eukaryotic cells.
C. They are all multicellular organisms.
D. They are made up of cells that lack a nucleus.
SUBMIT
Answers
Answer:
he presence of nucleus which contains genetic material and is enclosed by a nuclear membrane sets them apart from prokaryotic cells. All complex organisms are eukaryotic and they reproduce by mitosis or meiosis. Eukaryotic cells are typically much larger than those of prokaryotes.
Explanation:
Related Questions
How do I solve part A?

Answers
So the equation for Molarity is mole solute over Liter solution. The problem has already given you both to be able to find the molarity. 3.95mol of LiCl divided by 2.69 Liter of solution and you get 1.46M.
3
In which of the following states of matter are the particles very far apart so they can fill their containers?
A.
gases only
ОВ.
solids, liquids, and gases
solids and liquids only
C.
D.
solids only
Reset
Submit
Answers
Answer:
A. gases only
Explanation:
It is only in gases that the particles are very far apart so they can fill their contains.
There are four major states of matter which solid, liquid, gases and plasma.
Particles of a gas have very weak attractive forces between them. They do not have fixed volume nor shapeThey easily fill up any containers they are put into. Gases are compressible and moves randomly in a straight line. Liquids and solids do not fill up containers. Liquids although have weak forces but they are better attracted than gases. Solids have fixed and definite volume.So, option A is the perfect answer.
How many kilojoules are equivalent to 10 Joules?
A) 0.001 kJ
B) 0.01 kJ
C) 1000 kJ
D) 10,000 kJ
Answers
Answer:
It should be B: 0.01 kJ
Answer:
Hey...The answer is B) 0.01 kJ
How does ionic and covalent compounds use electrons?
Answers
Answer:
In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron donor and one electron acceptor. In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons.
They share electrons between them
A student adds 200.0g of C7H6O3 to an excess of C4H6O3, this produces C9H8O4 and C2H4O2. Calculate the percent yield if 231 g of aspirin (C9H8O4) is produced in an experiment.
___C7H6O3 + ___C4H6O3 ___C9H8O4 + ___C2H4O2
Answers
Answer:
Percent yield = 88%
Explanation:
Given data:
Mass of C₇H₆O₃ = 200.0 g
Actual yield of aspirin = 231 g
Percent yield = ?
Solution:
Chemical equation:
C₇H₆O₃ + C₄H₆O₃ → C₉H₈O₄ + C₂H₄O₂
Number of moles of aspirin:
Number of moles = mass/molar mass
Number of moles = 200.0 g/ 138.12 g/mol
Number of moles = 1.45 mol
Now we will compare the moles of aspirin with C₇H₆O₃.
C₇H₆O₃ : C₉H₈O₄
1 : 1
1.45 : 1.45
Theoretical yield of aspirin:
Mass = number of moles × molar mass
Mass = 1.45 g × 180.158 g/mol
Mass = 261.23 g
Percent yield:
Percent yield =( actual yield / theoretical yield )× 100
Percent yield = (231 g/ 261.23 g)× 100
Percent yield = 0.88 × 100
Percent yield = 88%
Which of the following is a covalent compound?
A BaCl2
(B) CHO
c) LiOH
D KNO3

Answers
Answer:
(B) CHO
Explanation:
A covalent bond is a bond type usually formed between non-metals that have little to no electronegativity differences between them.
Bonds between metals and non-metals are usually ionic in nature. All the other given choices are ionic and a combination of covalent bond.
CHO contains non-metals that are electronegative. There will be sharing of electrons between these atomic species and this will lead to a covalent bond.
Compare these two pictures to the graph above. In which of these situations did they use a catalyst (the left or right)? Why? In which of these two graphs will a reaction actually take place?
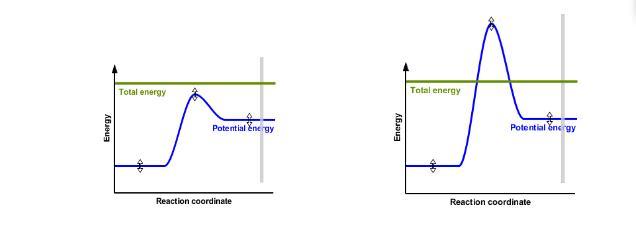
Answers
Answer:
Explanation:
The role of enzymes in a chemical reaction is to : Lower the activation energy while increasing the rate of reaction
The graph shown on the left is an uncatalyzed chemical reaction while the graph on the right is a catalyzed chemical reaction.
Catalyzed chemical reactions have lower activation energy and a faster rate of reaction while uncatalyzed chemical reaction has a higher activation energy and a slower reaction rate. because catalyst increases the rate of a chemical reaction by lowering its activation energy.
Hence we can conclude that The role of enzymes in a chemical reaction is to : Lower the activation energy while increasing the rate of reaction
(for a important test!! and use in your own words!!)
In one or two sentences, describe the connection between evaporation and a rainy climate.
Answers
Describe the relationship between evaporation and a rainy climate in one or two sentences.
How does evaporation explain itself?A liquid transforms into a gas during evaporation. It is easy to visualize when raindrops "vanish" from puddles on a hot day or when wet clothing dries in the heat. Instead of really dissipating in these situations, the liquid water is evaporating into a gas known as water vapor. Global evaporation takes place.
Why is it called evaporation?Evaporation occurs on surfaces. Because it happens when molecules with more kinetic energy from the top layer of the liquid escape into the air, evaporation is a surface phenomenon.
To know more about evaporation visit:
https://brainly.com/question/18800215
#SPJ1
what contains an electrolyte consisting of a base
Answers
NaOH contains an electrolyte consisting of a base. All soluble salts, acids, as well as bases dissolved inside a polar solvent.
What is electrolyte?An electrolyte is a material containing ions that conducts electricity through the motion of those ions but does not conduct electrons. All soluble salts, acids, as well as bases dissolved inside a polar solvent, including such water, fall into this category.
When the material dissolves, it splits into cations and anions which spread evenly throughout the solvent. There are other solid-state electrolytes. The term electrolyte describes the substance that's also dissolved in medicine and occasionally in chemistry. NaOH contains an electrolyte consisting of a base.
Therefore, NaOH contains an electrolyte consisting of a base.
To learn more about electrolyte, here:;
https://brainly.com/question/28699046
#SPJ9
which are the two major greenhouse gases?
A) CO2 and CH4
B) CH2O and CO
C) NO and NO2
D) O2 and H2O
correct answer is A)
Answers
What would be the primary species in solution at the first equivalence point in the titration curve for H2CO3 titrated with LiOH?
a. H2CO3 and OH-
b. HCO3-
c. H2CO3 and HCO3-
d. HCO3- and CO32-
e. CO32- and OH
Answers
The first equivalence point in the titration of H2CO3 (carbonic acid) with LiOH (lithium hydroxide) occurs when all the H2CO3 has been converted to its conjugate base HCO3-. At this point, the primary species in solution would be HCO3- and Li+. Therefore, the correct answer is b. HCO3-.
Carbonic acid is a carbon-containing compound which has the chemical formula H2CO3. Solutions of carbon dioxide in water contain small amounts of this compound. Its chemical formula can also be written as OC(OH)2 since there exists one carbon-oxygen double bond in this compound.
TO KNOW MORE ABOUT H2CO3 (carbonic acid) CLICK THIS LINK -
brainly.com/question/28175742
#SPJ11
If element X has 5 valence electrons, what would you expect it to do to fulfil its octet? pls help

Answers
Answer:
a
Explanation:
Answer:
Gain 3 electrons.
Explanation:
If element X have 5 valence electrons it will more than likely gain 3 electrons to fill its octet to become stable. Gaining the 3 electrons is much easier for the atom than if it were to give away it's 5 valence electrons. Elements with 5 valence electrons that could represent element X are:
NitrogenPhosphorusArsenicAntimonyWhat is the mass number of an atom that contains 80 electrons, 80
protons, and 100 neutrons?
Answers
Answer:
180
Explanation:
proton = 80
electron = 80
neutron = 100
Note:- the atomic number of an element is the number of protons/electrons in the atomic shell
(protons = electrons)
Mass number = number of protons/electrons + number of neutrons
MN= NP+ NN
MN = 80 + 100
MN= 180
I HOPE THIS HELPS
Where is the sun located?
What happenes to the suns core?
What three types of solar activity happenes in the photosphere on the sun?
Answers
1) The sun is a stat that is located in the middle of the solar system.
2) It gets squeezed together so lightly that four hydrogen nuclei combine to firm form helium atom. This is called nuclear fusion.
3) Solar flares, coronal mass ejection, high-speed solar wind and solar energetic particles.
you need a 3% solution of acid alcohol. how much hci will you need to add to 970 ml of 95% ethanol to obtain this concentration
Answers
To make a 3% solution of acid alcohol, you need to add 29.1 ml hydrochloric acid (HCl) to 970 ml of 95% ethanol.
To calculate how much HCl you need, you can use the formula:
Amount of HCl = Desired concentration x Volume of ethanol/100
Therefore, you will need 29.1 ml of HCl to make a 3% solution of acid alcohol:
Amount of HCl = 3 x 970/100 = 29.1 ml
You can also calculate the amount of HCl you need by using a ratio and proportion. Let x represent the amount of HCl you need.
95% ethanol : 3% acid alcohol :: 970 ml : x
By solving the proportion, you can determine that x is equal to 29.1 ml.
Thus, you will need 29.1 ml of HCl to make a 3% solution of acid alcohol when starting with 970 ml of 95% ethanol.
To know more about hydrochloric acid (HCl), refer here:
https://brainly.com/question/29105538#
#SPJ11
PLEASE HELP WITH MARK BRAINLIEST !!
Which of these is a special property of water? select all that apply
water has a high surface tension
none
water expands when it freezes
water molecules can stick together
Answers
Answer:
Water has high surface tension, and water molecules stick together
Explanation:
Answer: C and D
Explanation: Please give brainlyest
Earth has experienced many long periods of climatic cooling during which glaciers cover large areas of Earth. These periods are known as ice ages. Which of the following is a likely cause of ice ages?
A.
periodic changes in the shape of Earth's orbit
B.
the movement of the continents due to continental drift
C.
the shutdown of thermohaline circulation in the ocean
D.
periodic reversals to Earth's magnetic field
Answers
The phenomenon of the 'periodic changes in the shape of Earth's orbit' likely caused ice ages in the past (Option A).
What caused the ice ages?The ice ages are periods in the geological past in which temperature drastically decreased over thousands of years, which may be due to different factors such as Earth's orbital changes, volcanoes, modification in the ocean currents, amount of solar radiation, increase in the CO₂ and global warming, etc
In conclusion, the phenomenon of the 'periodic changes in the shape of Earth's orbit' likely caused ice ages in the past (Option A).
Learn more about ice ages here:
https://brainly.com/question/7387582
#SPJ1
Which example illustrates public scientific communication?
O A scientist collaborates with other scientists to plan an experiment.
O Ascientist sends a preview copy of a journal article to some colleagues.
O A federal government agency holds a press conference to describe the response to and the dangers of a new virus
epidemic,
A federal government agency publishes an article in a professional scientific journal detailing technical details of a
new virus
Answers
Answer:
D. A federal government agency publishes an article in a professional scientific journal detailing technical details of a new virus.
Explanation:
Public scientific communication is a way used by scientists to make the audience able to understand the basics of science to communicate an informed decision. There are several skills under public scientific communication that include connect with the public, talk to journalists, make science understandable, and understand the audience.
The best way to connect with the audience is to publish an article in a professional scientific journal because mostly journals are peer-reviewed having standard of quality.
A federal government agency informing about a new virus through an article in a professional scientific journal is the best way of public scientific communication as it will be for a specific audience along with a standard of quality.
Hence, the correct answer is "D".
Calculate the number of moles of neon gas in 8.15 atoms of neon gas

Answers
Answer:
13.5 moles Ne
Explanation:
Atoms --> Moles
1. Put the given number over 1
2. Multiply by 6.022x10^23 to get to moles
3. Round by sigfigs
(8.15x10^24 atom Ne(g)/1) (1 atom Ne(g)/6.022x10^23molesNe)=13.5337...
13.5337 --> 13.5 moles Ne
How many molecules of Na2O are in 24.6g? Show work!
Answers
Answer:
Explanation:
Na2O has 2 atoms of Sodium and 1 atom of Oxygen
So, the molecular mass of Na2O is:
(2 x Atomic weight of Na) + (1 x Atomic weight of O)
Atomic weight of Na = 22.99 g
Atomic weight of O = 16 g
Therefore, Molecular Mass of Na2O = (2x 22.99g) + (1 x 16g)
= (45.98 + 16)g = 61.98 g
Therefore 61.98 g of Na2O contains 6 x 10^23 molecules (Avogadro's number).
24.6 g of Na2O therefore contains:
(24.6/61.98) x 6 x 10^23 = Answer!
Which screw would create a strong hold?
A: The screw with 9 wraps.
B: The screw with 2 wraps.
C: The screw with 10 wraps.
D: The screw with 6 wraps.
Answers
Answer:
The screw with 10 wraps is the correct answer
Explanation:
The screw with 6 wraps would create a strong hold.
What do you mean by screw?The strongest member of the family of wood screws is a lag screw. It is a reliable and sturdy fastener that frequently has a square head or an externally powered hex drive.
In general, lag screws are significantly stronger and heavier than standard wood screws. They have a tapered point and coarse threads.
Thus, The screw with 6 wraps would create a strong hold.
To learn more about Screw, refer to the below link:
https://brainly.com/question/24168324
# SPJ5
11) A balloon weighing 144.85 g is filled with carbon dioxide and reweighed. The weight of the balloon plus gas is 153.77 g. The volume of the full balloon is 4.55 L. Calculate the density of carbon dioxide?
Answers
Answer:1.96 grams per liter
Explanation:To find density you have to divide mass by the volume d=m/v
Now you need to find the mass of the carbon dioxide by 153.77- 144.85= 8.92 grams
Finally all you do is 8.92/4.55= 1.96 grams per liter
A balloon weighing 144.85 g is filled with carbon dioxide and reweighed. The weight of the balloon plus gas is 153.77 g. The volume of the full balloon is 4.55 L the density of carbon dioxide will be 1.96 cm³.
What is density?
The density of any substance is the amount of the susbtance present at per unit cubic volume of the liquid and it is the ratio of the mass and the volume of the substance and unit will be centimeter cube.
The mass of carbon dioxide = 153.77 g - 144.85 g = 8.92
volume = 4.55 liters.
density = mass / volume
Substituting the value,
Density = 8.92 / 4.55 liters.
Density = 1.96 cm³.
Therefore, the density of carbon dioxide will be 1.96 cm³ if A balloon weighing 144.85 g is filled with carbon dioxide and reweighed. The weight of the balloon plus gas is 153.77 g. The volume of the full balloon is 4.55 L
Learn more about density, here;
https://brainly.com/question/15164682
#SPJ2
PLEASE HELP ASAP NO LINKS PLEASE
What is the force that keeps the planets in perfect orbit around the sun?
A) Velocity
B) Gravity
C) Centrifugal Force
D) Magnetism
Answers
Answer:
gravity
Explanation:
Gravity is the force that pulls us to the surface of the Earth, keeps the planets in orbit around the Sun and causes the formation of planets, stars and galaxies.
Hope this helped!!!
which of the following accurately describes the ph scale? which of the following accurately describes the ph scale? the ph scale runs from 0 (neutral) to 14 (most acidic), with 7 as an average acidity level. the ph scale runs from 0 (most acidic) to 14 (neutral), with 7 as an average acidity level. the ph scale runs from 0 (most basic) to 14 (most acidic), with 7 as a neutral. the ph scale runs from 0 (most acidic) to 14 (most basic), with 7 as a neutral.
Answers
Answer:
The pH scale measures acidity of a substance. known as potential of hydrogen, it varies from 0 to 14 with 7 being the pH value of a neutral solution. Below 7 shows the substance is acidic in nature and above 7 is alkaline in nature. pH 0-3 are considered strong acids while pH 4-6 are weak acids. pH 8-10 are weak alkalines and pH 11-14 are strong alkalines. This is a general trend and there may be exeptions especially if the substance has a negative pH. However, it would not be covered likely unless you are doing university chemistry.
How could the experimental set up result in an artificially high product yield? Briefly explain.
Answers
Percent yield is defined as the ratio of the actual yield to the theoretical yield. It is expressed in the form of percentage.
Percent Yield is defined as the ration when the actual yield divided by the theoretical yield times 100. The actual yield of a chemical reaction may be less than the theoretical yield. The percent yield can be expressed as the ratio of the actual yield to the theoretical yield expressed as a percentage. The theoretical yield is defined as the maximum amount of product that can be formed from the given amounts of reactants. The actual yield is defined as the amount of product that is actually formed when the reaction is carried out in the laboratory. It is a measure of the quantity of moles of a product formed in relation to the reactant consumed obtained in a chemical reaction.
To learn more about Percent yield
https://brainly.com/question/24287647
#SPJ4
0.265g of an organic compound produced on evaporation 102cm cube of vapour at 373K and 775mmHg. Percentage composition of the constituent elements are 92.24% C and 7.76% H. Find the molecular mass and molecular formula of the composition.
Answers
Answer:
No results found for 0.265g of an organic compound produced on evaporation 102cm cube of vapour at 373K and 775mmHg. Percentage composition of the constituent elements are 92.24% C and 7.76% H. Find the molecular mass and molecular formula of the composition..
Results for 0.265g of an organic compound produced on evaporation 102cm cube of vapour at 373K and 775mmHg Percentage composition of the constituent elements are 92.24 C and 7.76 H Find the molecular mass and molecular formula of the composition
Answer:
Using PV=nRT
since \(n. \: no \: of \: moles= \frac{mass}{molar \: mass} \)PV= nRT
P=775mmhg/760mmhg
P=1.01atm
so,n=PV/RT
n= (1.01*0.102)/(0.0821*373)
n= 0.00339mole
n =mass/ molecular mass
molecular mass =0.265/0.00339
molecular mass= 78.17
empirical formular = C. H
C= 92.24. 92.24/12= 7.688
H= 7.76. 7.76/1=7.76
therefore Empirical formula is CH
(CH)n=78.16
(12+1)n=78.14
n=78.14/13
n= 6.01
therefore, molecular formula is
C6H6
What is the term for what happens when light travels from one medium to another?
Answers
Answer:
is there any answer choices????
Answer:
The answer is Refraction
2. Consider dimethyl ether at 300 K which has an angle averaged radius of 0.25 nm. a) Calculate its collision frequency at 1 bar and 1 Pa. b) Calculate its decomposition rate constant k (CH3)2CO produ
Answers
a) The collision frequency of dimethyl ether can be calculated using the kinetic theory of gases. The collision frequency is given by the equation:
\(\[\text{{Collision frequency}} = \frac{1}{4} \sqrt{\frac{8 \cdot k \cdot T}{\pi \cdot m}}\]\)
where k is the Boltzmann constant, T is the temperature in Kelvin, and m is the mass of dimethyl ether molecule. Given that the angle-averaged radius of dimethyl ether is 0.25 nm, we can calculate the mass of the molecule using its density or molar mass.
b) To calculate the decomposition rate constant of (CH3)2CO, we need additional information such as the reaction mechanism and reaction conditions. The rate constant for a chemical reaction depends on factors like temperature, activation energy, and the presence of catalysts. Without these details, it is not possible to calculate the decomposition rate constant accurately.
In conclusion, the collision frequency of dimethyl ether at a specific temperature can be calculated using the kinetic theory of gases. However, to calculate the decomposition rate constant of (CH3)2CO, additional information about the reaction conditions and mechanism is needed.
To know more about Molar Mass visit-
brainly.com/question/31545539
#SPJ11
Put the following chromatography solvent in order of polarity as a chromatography solvent: acetone, npropanol, water, hexane, diethyl ether, methanol. 2) You will be doing chromatography with a mixture of n-propanol and water. Would it make sense to add 5) Two cars full of people took a day trip. Car A had a lot of little kids and stopped at lots of rest stops and gas stations to give them a break. Car B had just adults in it, and only stopped once. Of course Car B got to the destination first. Explain how this in an analogy for the chromatography you are doing in this experiment help please
Answers
LEAST TO MOST POLAR- hexane,diethyl ether,acetone,n-propanol,water ,methanol
Considering the problem we can say that the kids are compared to be more polar or smaller molecular weight compounds. The more polar compounds interact with silica more just as the kids bus stopped at every point and reached the destination late. Whereas, the adult bus is compared to be less polar compounds or high molecular weight which interacts less and reaches the destination faster.
Thus more polar compounds reaches the destination late whereas less polar compound reaches first.
find more:-https://brainly.com/question/13765692?referrer=searchResults
#SPJ4
The modern day three little pigs will need to choose from the following materials to build their energy efficient home: Vibranium, Beskar, and Gundarium
order for the three little pigs to be successful in building an insulated home that loses as little heat as possible, they will need to choose the materal with ta
highest specific heat, Cp-
Vibranium
Answers
Choosing the material with the highest specific heat capacity is ideal and true.
What is Specific heat capacity?This is defined as the quantity of heat needed to raise the temperature per unit mass.
Insulators have a higher specific heat capacity as it takes more time for them to absorb heat thereby making it ideal in building an insulated home.
Read more about Specific heat capacity here https://brainly.com/question/26866234