Rank the following compounds in order of basicity: pyridine, 3-nitropyridine, 3-chloropyridine.
Answers
The order of basicity for these compounds is:
Pyridine.3-nitropyridine.3-chloropyridine.The basicity of a compound refers to its ability to donate a pair of electrons. In general, the more electronegative an atom is, the less basic a compound will be. The electronegativity of the atoms in a compound can be used to predict its basicity.
In the case of the compounds you listed, pyridine is the most basic because it has the least electronegative atoms. 3-nitropyridine is less basic than pyridine because it has a nitro group (-NO2), which is more electronegative than a hydrogen atom. Finally, 3-chloropyridine is the least basic because it has a chlorine atom, which is even more electronegative than a nitro group.
Learn more about bacity, here https://brainly.com/question/15347758
#SPJ4
Related Questions
m
Which two types of energy are formed by the transformation shown in the
photo?
O A. Chemical energy - thermal energy
B. Thermal energy - electrical energy
C. Thermal energy - chemical energy
D. Chemical energy - electromagnetic energy
Answers
Answer:
D
Explanation:
Hope it helps
#CarryOnLearning
What is the valency of carbon in CH 4?
Answers
The valency of carbon in methane (CH₄) is Four.
Valency is simply defined and is equal to the number of electrons gained, lost or shared by an atom of an element to achieve the nearest Nobel gas configuration which is completing eight, eighteen etc. electrons in its last shell.
The number of atoms of other elements with which one atom of an element will combine is decided by the valency of that element.
In the given question, as carbon has four valence electrons in its last shell so its valency becomes four.
Similarly, If you were asked to find the valency of hydrogen in CH4 you can tell that it is one because it has one electron placed in its last shell.
Learn more about Valence electrons here:
https://brainly.com/question/12744547
#SPJ4
The reaction below can be identified as a _______________ reaction. 3H2 + N2 → 2NH3
Answers
An illustration of a combination reaction is the one that follows:
\(3H_2+N_2 - > 2NH_3\).
What is Combination Reaction?When two or more compounds (reactants) combine to form a single substance (product), this is referred to as a combination reaction. This reaction also generates a large amount of energy in the form of heat in addition to the formation of new bonds and the products that arise.
Two reactants combine to produce one product in a combination reaction. Oxygen and halogens are very reactive, making them likely to mix with other elements to create new compounds. When two or more reactants combine to produce a single product, this is referred to as a combination reaction.
To know more about combination reaction, visit:
https://brainly.com/question/15092182
#SPJ1
how many mercury atoms are contained in 0.37 moles of mercury?
Answers
Which of the following are spectator ions in the equation below? Select all that apply
Pb(C₂H302)2(ag) + 2 LiCl(ag) 2 LIC₂H302(ag) + PbCl2(s)
Pb2+(aq)
Li+(aq)
Cl-(aq)
C2H3O-2(aq)
I hope i entered the answer options right
Answers
which is the correct order of ease of carbon dioxide production by heating the group II metal carbonates?
Answers
Which is the correct order of ease of carbon dioxide production by heating the group II metal carbonates?
A) \(BaCO_{3} > SrCO_{3} > CaCO_{3} > MgCO_{3}\)
B) \(MgCO_{3} > CaCO_{3} > SrCO_{3} > BaCO_{3}\)
C) \(CaCO_{3} > MgCO_{3} > BaCO_{3} > SrCO_{3}\)
D) \(SrCO_{3} > BaCO_{3} > CaCO_{3} > MgCO_{3}\)
Answer:
Explanation:
The correct option is A:
\(BaCO_{3} > SrCO_{3} > CaCO_{3} > MgCO_{3}\)
On the periodic table, carbonates required stronger heating as one does down the groupings in order for them to decompose. The implication is that the stability of compounds will increase from \(MgCO_{3}\) to \(BaCO_{3}\) .
So the carbonates will be stable according to the following order:
\(BaCO_{3} > SrCO_{3} > CaCO_{3} > MgCO_{3}\), thus making B the correct answer.
Cheers
What are the 7 main properties of matter?
Answers
Answer:Color, Density, Volume, Mass, and Boiling point. These are 5 3 more to go!
Explanation:
Color (intensive), Density (intensive), Volume (extensive), Mass (extensive) , Boiling point (intensive), Melting point (intensive) are the 7 main properties of matter .
What are properties of matter?
Any attribute that can be measured, including an object's density, color, mass, volume, length, malleability, melting point, hardness, odor, temperature, and more, is referred to as a property of matter.
Short answer: What is the matter?
The term "matter" refers to a substance that is composed of several kinds of particles, occupies space, and has inertia. The different kinds of particles each have a unique mass and size, according to the fundamentals of current physics.
The electron, proton, and neutron are three of the most prevalent instances of material particles.
Learn more about Matter
brainly.com/question/28487167
#SPJ4
9.0 mol Na2S can from 9.0 mol CuS and 8.0 mol CuSO4 can form 8.0 mol Cus.
What mass of Cus forms during the reaction?
Cus; 95.62 g/mol

Answers
Answer:
765.0 grams CuS
Explanation:
The limiting reagent is the reactant which completely reacts before the other reactant(s) is used up. When 9.0 moles Na₂S and 8.0 moles CuSO₄ react, it appears that CuSO₄ is the limiting reagent. You can tell because it results in the production of less product.
You can determine the mass of CuS by multiplying the moles by the molar mass. It is important to arrange the ratio in a way that allows for the cancellation of units.
Molar Mass (CuS): 95.62 g/mol
8.0 moles CuS 95.62 g
------------------------- x ----------------------- = 765.0 grams CuS
1 mole
what is the chemical formula of the compounds
(a) calcium fluoride
(b) aluminum carbonate
(c) calcium hydrogen sulphate
(d) potassium nitrate
Answers
Explanation:
what is the chemical formula of the compounds?Answer: B Aluminum Carbonate
#CarryOnLearning.
Explain how the graph above can be used to find the half-life of an isotope.
Explain why the limit of radiocarbon dating using carbon-14 is approximately 60,000 years (10 half-lives).

Answers
The half life is the time taken for only one of the half of the radioactive substance to remain.
What is half life?The half life is the time taken for only one of the half of the radioactive substance to remain. We can see the half life by looking at the graph and observing the point at which the sample decreases to half its original number.
Since the half life of carbon-14 is 5700 years, after ten half lives, almost 60000 years has elapsed thus there is little or no carbon-14 left. As a result of this carbon-14 can not be used if the sample is over 60000 years old.
Learn more about half life:https://brainly.com/question/24710827
#SPJ1
Which of the compounds below will ionize in water? Check all that apply. (PLEASE INCLUDE AN EXPLANATION OF YOUR PROCESS!)
A) H2SO4
B) NH3
C) NaCl
D) SO2
E) MgBr2

Answers
The compounds that are able to ionize completely in water are;
Sulfuric acidSodium chloridemagnesium bromideWhat is an ionic compound?We define an ionic compound as a compound that is able to dissolve in water. We know that the dissolution occurs because the the molecules of water is able to surround the ions in the compound and then pull them apart.
This implies that we have to start looking at the compounds that are able to be dissolved in water quite easily by the process of hydration in which the ions in the compound are pulled apart by water. These, like I said are the compounds that we call ionic compounds.
The compounds that we look out for are the acids and other ionic compounds and we can see that they are part of the list that we can see in the question listed above.
Learn more about ionic compounds:https://brainly.com/question/9167977
#SPJ1
what is the conjugate base of HC6H6O6-
Answers
Answer:
the conjugate base of the compound is
C6h6O2/6-
Explanation:
subscript
The conjugate base of \(\rm HC_6H_6O_6^-\; is \; C_6H_6O_6^-\).
What are conjugate acids and bases?Conjugate acid is the acid that donates a hydrogen ion to a base, conjugate acid contains a hydrogen ion.
The base that gains a hydrogen ion from a conjugate acid is called the conjugate base.
The acid is NH4 converts into a conjugate base NH3-
When a conjugate acid donates a hydrogen ion, it converts into the conjugate base.
Thus, the conjugate base is \(\; C_6H_6O_6^-\)
Learn more about the conjugate base
https://brainly.com/question/10468518
What do you call a substance that can only be separated into two or more simpler substances using chemical changes?
Answers
Answer:
Compounds are substances composed of two or more elements chemically combined that can be separated into simpler substances only by chemical means. Water, for example, is a compound because pure water is composed of only H2O molecules.
Pls choose me as brainliest!
physical and chemical proportys
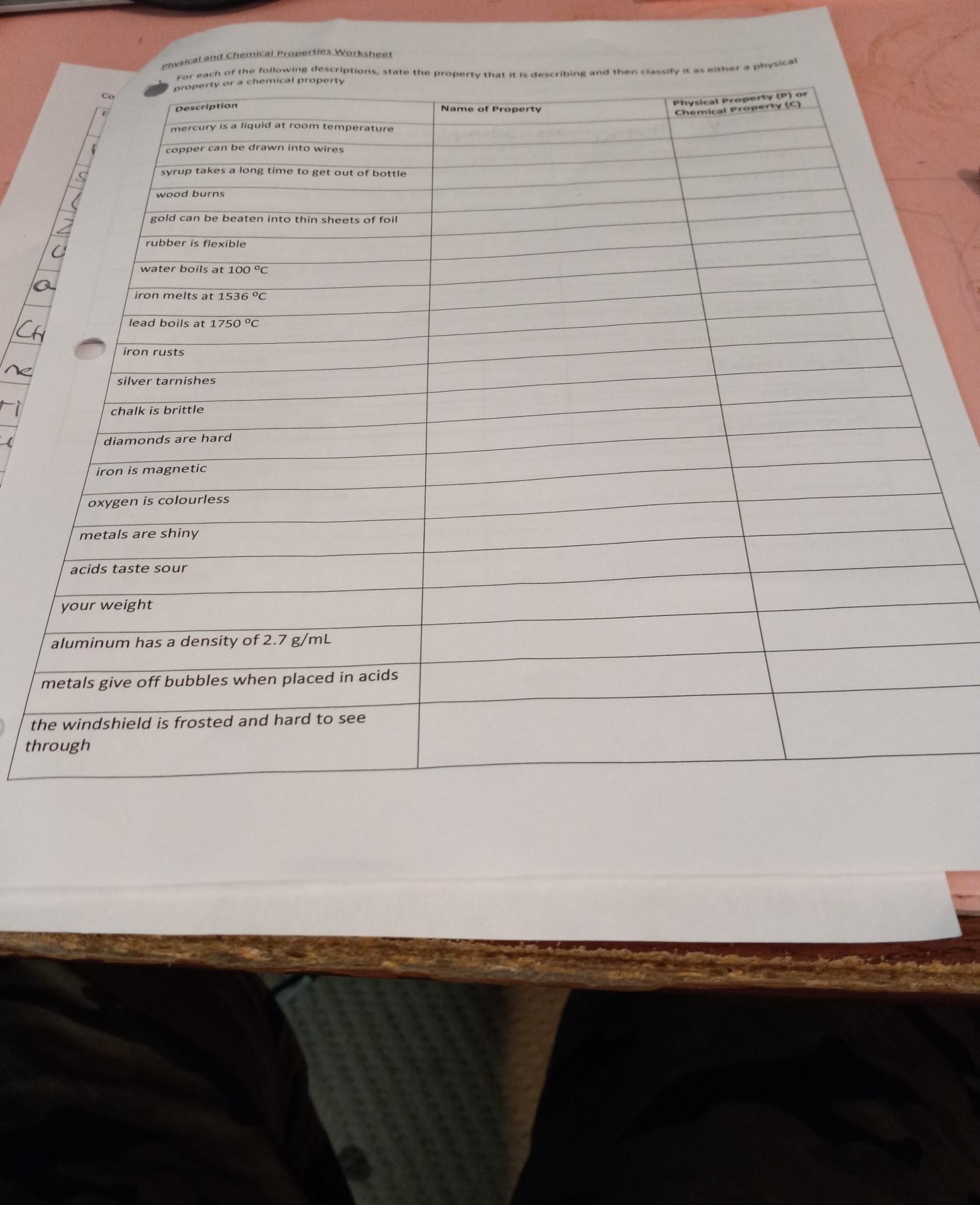
Answers
The physical and the chemical properties of matter are listed below.
What are the examples of physical and chemical properties?While the entire page is not clear, I have an idea of what you are trying to ask and I would show you the physical and the chemical properties of matter.
Physical properties are characteristics of a substance that can be observed or measured without changing the substance's chemical composition. Examples of physical properties include:
Density
Color
Boiling point
Melting point
Hardness
Solubility
Electrical conductivity
Optical properties (such as refractive index)
Chemical properties are characteristics of a substance that describe its ability to undergo chemical reactions and form new substances. Examples of chemical properties include:
Reactivity with other substances
Combustibility
Oxidation state
Acidity or basicity (pH)
Toxicity
Flammability
Learn more about matter:https://brainly.com/question/28487167
#SPJ1
Which element has one energy level?
a) sodium
b) boron
c) potassium
d) helium
Answers
The element with a single energy level from the list is helium. Option D.
What is energy level?
An energy level is a specific region of space around the nucleus of an atom where electrons can exist with a certain amount of energy.
The number of energy levels an atom has depends on the number of electrons it has, as well as the atomic structure of the element.
Helium, with an atomic number of 2, has two electrons in total. These two electrons occupy the first and only energy level that helium has, which is known as the K-shell.
In contrast, elements with more than two electrons, such as sodium, boron, and potassium, have multiple energy levels or shells, each of which can hold a specific number of electrons.
More on energy levels can be found here: https://brainly.com/question/17396431
#SPJ1
If an atom has an atomic number of 17 and a mass of 35, how many neutron does it have?
Will give brainliest
Answers
Answer:
18
Explanation:
(atomic number = protons) mass minus protons= neutrons.
Where does the energy required to break the interactions between butane molecules come from when butane boils?
Answers
Answer:
The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In contrast to intramolecular forces, such as the covalent bonds that hold atoms together in molecules and polyatomic ions, intermolecular forces hold molecules together in a liquid or solid. Intermolecular forces are generally much weaker than covalent bonds. For example, it requires 927 kJ to overcome the intramolecular forces and break both O–H bonds in 1 mol of water, but it takes only about 41 kJ to overcome the intermolecular attractions and convert 1 mol of liquid water to water vapor at 100°C. (Despite this seemingly low value, the intermolecular forces in liquid water are among the strongest such forces known!) Given the large difference in the strengths of intra- and intermolecular forces, changes between the solid, liquid, and gaseous states almost invariably occur for molecular substances without breaking covalent bonds.
Explanation:
im not sure this is what your looking for but i found this
The volume of a gas is 0.9 L at 273 K and 1.2 atm. What pressure will the gas occupy if the temperature is raised to 325 K and the volume is raised to 1.8 L?
Answers
when the temperature is raised to 325 K and the volume is increased to 1.8 L, the gas will occupy a pressure of approximately 1.08 atm.
To solve this problem, we can use the combined gas law equation, which relates the initial and final conditions of a gas when the amount of gas remains constant.
The combined gas law equation is given as:
(P₁ * V₁) / T₁ = (P₂ * V₂) / T₂
where P₁, V₁, and T₁ represent the initial pressure, volume, and temperature, respectively, and P₂, V₂, and T₂ represent the final pressure, volume, and temperature, respectively.
Given
Initial pressure (P₁) = 1.2 atm
Initial volume (V₁) = 0.9 L
Initial temperature (T₁) = 273 K
Final volume (V₂) = 1.8 L
Final temperature (T₂) = 325 K
We need to find the final pressure (P₂).
Substituting the given values into the combined gas law equation, we have:
(1.2 atm * 0.9 L) / 273 K = (P₂ * 1.8 L) / 325 K
Now, we can solve for P₂:
P₂ = (1.2 atm * 0.9 L * 325 K) / (273 K * 1.8 L)
= 1.08 atm
for more questions on temperature
https://brainly.com/question/2339046
#SPJ8
If a sulfur atom gained 3 protons what atom would be formed?
Answers
Answer:
The stable ion the sulfur would form is the sulfide ion,
Explanation:
When any atom accept electrons it converts into negative ion/an anion. So it will change into Z 3− after gaining 3 electrons.
Sulfur is an elemental atom that has an atomic number of 16 and an atomic mass of 32.065 amu. If the sulfur atom gains three protons then potassium will be formed.
What is a proton?A proton has been a sub-atomic particle that has been known to carry a positive charge and are tend to remain inside the nucleus with the neutrons bonded together by the nuclear force.
The proton of the atom is the characteristic property of the element in the periodic table. They are the sole sub-atomic particle that gives the atomic number of the elemental atom.
The sulfur atom has the atomic number 16 which means it has 16 protons, so if three protons are added to it then the number f protons become 19 which is an atomic number of the potassium (K) element.
Therefore, a potassium element is formed.
Learn more about protons, here:
https://brainly.com/question/24508299
#SPJ2
Give the symbol balanced equation for the reactions below. Ensure states are used.
a) Carbonic acid forming when a hydrogen ion reacts with a bicarbonate ion in a reversible reaction.
Answers
Answer:
\({ \rm{2H {}^{ + } _{(aq)} + CO {}^{2 - } _{3(aq)} \: \: \: {}^{ { \huge{\dashrightarrow} }} _{ \huge{ \dashleftarrow}} }} \: \: { \rm{H _{2} CO _{3(aq)} }}\)
In which situation would hydrogen bonding be present?
A. When hydrogen exists as an ion in solution
B. When hydrogen is attached to C, S, or P
C. When hydrogen is attached to N, F or o
D. When hydrogen atoms bond together to form H2
Answers
Answer:
C
Explanation:
hydrogen Bond exists between ammonia, hydrogen flouride and water so the answer is C
what's the word equation for table salt?
Answers
Answer:
Have Nice Day
Explanation:
2NaCI is the answer
a particle-level diagram of a metallic element is shown above. typically, metals are both malleable and ductile. the best explanation for these properties is that the electrons involved in bonding among metal atoms are select one: a. equally shared and form highly directional bonds b. unequally shared and form nondirectional bonds c. unequally shared and form highly directional bonds d. equally shared and form nondirectional bonds
Answers
a particle-level diagram of a metallic element is shown above. typically, metals are both malleable and ductile. the best explanation for these properties is that the electrons involved in bonding among metal atoms are equally shared and form nondirectional bonds
Because the charge is uniform throughout, ionic bonds are non-directional. Another ion surrounds an ion in all directions. However, covalent and coordinate bonds can only form from one side, making them directed.
However, are metallic bonds non-directional given that they differ from covalent, ionic, and coordinate bonds. The only thing a metallic bond is is the attraction between an electron cloud and a metal. Therefore, this has no direction at all. Because the charge is uniform in all directions, ionic bonds are non-directional. Another ion surrounds an ion from every angle. However, covalent and coordinate bonds can only form from one side, making them directed.
learn more about electron cloud here;
https://brainly.com/question/1416504
#SPJ4
mr. turner would like to fill his alprazolam 1mg with a sig of 1 q8h, what is wrong with this prescription?
Answers
The prescription for Mr. Turner's alprazolam 1mg with a sig of 1 q8h is missing important information such as the duration of the treatment and the total number of pills to be dispensed.
Without this information, the patient may not know how long to take the medication and may run out before the treatment is complete. Additionally, the frequency of 1 q8h (once every 8 hours) may be too frequent for alprazolam and could result in an overdose or other adverse effects. It is important for the prescriber to provide clear and accurate instructions to ensure safe and effective use of the medication.
Mr. Turner's prescription for alprazolam 1mg has a sig of 1 q8h, which means he should take 1 tablet every 8 hours. There is nothing inherently wrong with this prescription, as long as it has been prescribed by a healthcare professional and is appropriate for Mr. Turner's medical condition.
Alprazolam is typically used to treat anxiety and panic disorders, and the dosage depends on the patient's individual needs and response to the medication.
Visit here to learn more about medication : https://brainly.com/question/11098559
#SPJ11
Mr. Turner's prescription for alprazolam 1mg with a sig of 1 q8h has the following issue:
The term "sig" refers to the directions for use, which in this case is "1 q8h." This means Mr. Turner should take 1 tablet every 8 hours. However, alprazolam is a benzodiazepine used to treat anxiety and panic disorders, and its dosing frequency is typically not as high as every 8 hours. The standard dosing frequency for alprazolam is usually 2-3 times a day.
The wrong aspect of this prescription is the dosing frequency (1 q8h) which may lead to potential overuse or increased side effects. It is essential to consult with a healthcare professional to determine the appropriate dosing and frequency for Mr. Turner's needs.
To know more about alprazolam :
https://brainly.com/question/29060963
#SPJ11
Write the names of the reactants and products of photosynthesis and chemosynthesis.
Answers
Whole reaction for photosynthesis:
6H2O +6CO-> C6H12O6 + 6O
HNO3(aq)+H,0(1)+2NO(g) what is the total number of nitrogen atoms?
Answers
Answer:
3n+2n=5n 5 nitrogen atoms
how do you make up 275 ml of a 4.6 m solution of hcl (gmw=36.46 g/mol)?
Answers
To make up the 275 ml of a 4.6 M solution of the HCl we need 45.93 g of the HCl.
The molarity of the HCl solution = 4.6 M
The volume of the HCl solution = 275 mL
The molarity is expressed as :
Molarity = moles / volume in L
Moles = molarity × volume
Moles = 4.6 × 0.275
Moles = 1.26 mol
The number of the moles is as :
Number of the moles = mass / molar mass
Where
The molar mass = 36.46 g/mol
Mass = number of moles × molar mass
Mass = 1.26 × 36.46
Mass = 45.93 g
To learn more about molarity here
https://brainly.com/question/8732513
#SPJ4
why was it necessary to titrate the cold solution in the ice bath, but not necessary to titrate the hot solution in the hot bath?
Answers
The solubility of the Ca(OH)2 would be greatly increased since the lower temperatures would result in a slower titration and the solution would have time to warm up.
What is titration?
When a reaction reaches neutralisation, which is sometimes signalled by a colour change, titration is the steady addition of one solution with a known concentration (referred to as a titrant) to a known volume of another solution with an unknown concentration. To be a primary or secondary standard, the so-called titrant must meet the appropriate criteria. Titration is a method for figuring out a solution's concentration in a wide sense.
One of the processes in the manufacture of silver nanoparticles involves gently combining sodium borohydride with silver nitrate in an ice bath to create yellow colloidal silver.
Since the titration would proceed more slowly at the lower temperatures and the solubility of the Ca(OH)2 would increase dramatically as the solution warmed, it was necessary to titrate the cold solution in the ice bath.
Learn more about titration from given link
https://brainly.com/question/13307013
#SPJ4
Calculate the ph of a 1 l solution containing:
a. 20ml of 5 m koh.
a. 10ml of 0.1 m glycine and 20ml of 2m hcl
c. 5ml of 2 m acetic acid and 5 grams of sodium acetate (82g/mol).
Answers
(a) pH of the 1 L solution containing 20 mL of 5M KOH is 11.699.
(b) pH of the 1 L solution containing 10 mL of 0.1 M glycine and 20 mL of 2M HCl is 1.398.
(c) pH of the 1 L solution containing 5 mL of 2M acetic acid and 5 g of sodium acetate is 3.87.
a. Calculation of pH of 20 ml of 5M KOH:
Concentration of KOH solution = 5 M
Volume of KOH solution = 20 mL = 0.020 L
Polarity of KOH = (5 mol) / (1000 mL) = 0.005 mol/mL
Number of moles of KOH in 20 mL = 0.020 L × 0.005 mol/mL = 0.0001 mol
Concentration of OH- ions in 20 mL of KOH solution = (0.0001 mol) / (0.020 L) = 0.005 M[pOH = -log (OH-)]pOH = -log (0.005) = 2.301
pH + pOH = 14pH = 14 - 2.301 = 11.699
pH of the 1 L solution containing 20 mL of 5M KOH is 11.699.
b. Calculation of pH of 10 mL of 0.1 M glycine and 20 mL of 2M HCl:
Volume of glycine solution = 10 mL = 0.010 L
Volume of HCl solution = 20 mL = 0.020 L
Polarity of glycine = (0.1 mol) / (1000 mL) = 0.0001 mol/mL
Number of moles of glycine in 10 mL = 0.010 L × 0.0001 mol/mL = 0.000001 mol
Concentration of H+ ions in 20 mL of 2M HCl solution = 2 MC = c(H+) + c(glycine)C = 2 MC(H+) = 2 mol/L
Number of moles of H+ ions in 20 mL = 0.020 L × 2 mol/L = 0.040 mol
Concentration of H+ ions in 1 L solution = (0.040 mol + 0.000001 mol) / 1 L = 0.040001 mol/L
pH = -log (0.040001) = 1.398
pH of the 1 L solution containing 10 mL of 0.1 M glycine and 20 mL of 2M HCl is 1.398.
c. Calculation of pH of 5 mL of 2M acetic acid and 5 g of sodium acetate (82g/mol):
Molar mass of sodium acetate, NaC2H3O2 = 82 g/mol
Mass of sodium acetate = 5 g
Number of moles of sodium acetate = (5 g) / (82 g/mol) = 0.06098 mol
Volume of acetic acid solution = 5 mL = 0.005 L
Volume of sodium acetate solution = 0.005 L + (0.06098 mol / 2 M) = 0.03549 L
Polarity of acetic acid = (2 mol) / (1000 mL) = 0.002 mol/mL
Number of moles of acetic acid in 5 mL = 0.005 L × 0.002 mol/mL = 0.00001 mol
C = c(CH3COOH) + c(CH3COO-)c(CH3COOH) = c(CH3COO-)Ka = 1.8 × 10^-5Ka = ([H+][CH3COO-]) / [CH3COOH]
Since [CH3COOH] = [CH3COO-] + [H+] and [H+] is very small as compared to [CH3COO-], [H+] can be neglected.[H+] = sqrt(Ka × c(CH3COOH))= sqrt (1.8 × 10^-5 × 0.00001) = 0.000134mole/L
pH = -log (0.000134) = 3.87
pH of the 1 L solution containing 5 mL of 2M acetic acid and 5 g of sodium acetate is 3.87.
Know more about pH:
https://brainly.com/question/2288405
#SPJ11
you make a solution of 0.1 m naoh and accidentally spill the solution, covering your gloved hand and onto your arm. your wrist was exposed, and the solution came into direct contact with your skin. what should you do?
Answers
If you accidentally get NaOH solution on your skin, take the following laboratory actions:
tell the teacher right awaywash the affected area with waterCertain hazards can occur in laboratories and require immediate action and rapid containment.
According to this question, the NaOH solution is inadvertently splashed onto the skin. NaOH is irritant so you should wash it with water
If you accidentally get NaOH solution on your skin, take the following actions:
tell the teacher right awaywash the affected area with waterFor more information on lab actions, please visit:
Brainly.com/question/4783702
#SPJ4