Summarize the main issues that are facing social security and medicare, and describe one solution that you would use to address these issues?
answer: because the united states' population is aging, this means that costs are rising for programs that assist senior citizens. because funding is the major challenge that must be solved, i would consider changing the retirement age. as citizens live longer lives, they may be able to have longer careers. however, this would be a difficult solution.
Answers
Social Security and Medicare are two programs that are facing significant challenges due to the aging population of the United States. As more people retire and require benefits, the cost of these programs is rising rapidly. Additionally, there are concerns about the long-term solvency of these programs, as their funding sources are limited.
One of the main issues facing Social Security is the fact that the trust fund that supports it is projected to run out of money by 2035. This means that the program will only be able to pay out about 75% of benefits at that time unless changes are made. Similarly, Medicare is facing funding challenges due to the rising costs of healthcare and an increasing number of beneficiaries. However, changing the retirement age is a difficult solution that would need to be implemented carefully. It could impact low-income workers and those in physically demanding jobs, who may not be able to work for longer periods of time. Additionally, it may be unpopular with some segments of the population who feel entitled to retire at a certain age.
Overall, Social Security and Medicare are facing significant challenges that require creative solutions. While changing the retirement age could be one possible solution, it must be done with caution and consideration for the needs of all Americans.
To know more about Social Security visit:-
https://brainly.com/question/32221263
#SPJ11
Related Questions
(don’t need to answer all the questions i just need help with these!)
1) how many grams of iron are produced when 450. grams of iron (III) oxide react?
2) how many grams of water will be produced when 0.0155 moles of hydrogen gas completely react with Iron (III) oxide?
3) How many moles of mercury (II) oxide are needed to produce 12 grams of mercury?
4) How many moles of oxygen gas will be produced from 0.03 mol mercury (II) oxide?
5) How many moles of AlPO4 are produced when 0.27 of K3PO4 were used?
6) What is the mass of K3PO4 are needed to produce 2.04 moles of KNO3?
Answers
Answer:
Explanation:
1) Fe2O3 + 3 H2 -----> 2 Fe + 3 H2O Number of moles of Iron (III)oxide = 450 g / 159.69 g/mol = 2.82 mole
2) 0.0155 moles of hydrogen gas
3) oxide needed to produce 125 of oxygen is7.8 moles
4) 216.5894
5) K3PO4 and KNO3
9
Calcium carbonate reacts with dilute hydrochloric acid according to the equation shown.
CaCO3 + 2HCl → CaCl2 + CO₂ + H₂O
10g of calcium carbonate is reacted with 100 cm³ of 1 mol/dm³ hydrochloric acid.
mole calculation is needed
The following statements are made.
1
2
3
4
1.2 dm³ of carbon dioxide is formed.
5.6g of calcium chloride is formed.
4.8 g of carbon dioxide is formed.
No calcium carbonate is left when the reaction is completed.
Which statements about the reaction are correct?
A 1 and 2
B 1 and 4
C 2 and 3
D 3 and 4
Answers
4.8 g of carbon dioxide is formed and No calcium carbonate is left when the reaction is completed statements are correct.
Calculation and Explanation :CaCO3 has a molar mass of 40+12+316.
CaCO3 has a molar mass of 100 g/mol.
CaCl2 has a molar mass of 40 + 235.5.
CaCl2 has a molar mass of 111 g/mol.
100 g of CaCO3 and 111 g of CaCl2 are produced per mole.
Assume that x g of CaCl2 is created for every 10 g of CaCO3.
Therefore,
CO2 has a molar mass of 12 + 162.
CO2 has a molar mass of 44 g.
100 g of CaCO3 yield 44 g of CO2 per mole, or one mole.
Assume 10 g of CaCO3 results in y g of CO2.
Therefore,
y = 4.8 g 4.8 g of CO2 are created, meaning that one statement is false and three are true.
So we can say that the option D is correct . When the reaction is finished, the assertions that read "4.8 g of carbon dioxide is produced" and "No calcium carbonate is left" are true.
To know more about Calcium carbonate please click here ; https://brainly.com/question/1990063
#SPJ9
If 6.00 moles of CaO is combined with CO2, how many grams of CaCO3 would be formed
Answers
600.54 g of CaCO3 would be formed
The chemical equation in balanced form for the reaction between CaO and CO2 to form CaCO3 is
CaO + CO2 -> CaCO3
Here 1 mol of CaO reacts with 1 mol of CO2 to form 1 mol of CaCO3
Therefore 6.00 moles will combine with 6.00 moles of CO2 to form 6.00 moles of CaCO3.
The molar mass of CaCO3= 100.09 g/mol
Number of moles of CaCO3=6.00 moles
we know that,
Number of moles of CaCO3= mass of CaCO3/molar mass of CaCO3
Therefore mass of CaCO3 =Number of moles of CaCO3* molar mass of CaCO3
Mass of CaCO3=6.00moles*100.09g/mol
= 600.54g
To learn more about questions related to chemical equations refer to:
https://brainly.com/question/14562573?
Mass of CaCO3 = 600.54g
Explanation:
The balanced chemical equation for the reaction between CaO and CO2 to form CaCO3 is:
CaO + CO2 → CaCO3
From the balanced equation, we can see that 1 mole of CaO reacts with 1 mole of CO2 to produce 1 mole of CaCO3.
To determine how many grams of CaCO3 will be formed from 6.00 moles of CaO, we need to use the molar mass of CaCO3, which is 100.09 g/mol.
The calculation is as follows:
6.00 moles of CaO × (1 mole of CaCO3 / 1 mole of CaO) × (100.09 g/mol) = 600.54 g
Therefore, 600.54 grams of CaCO3 will be formed from 6.00 moles of CaO.
what is the concentration, in m/v percent, of a solution prepared from 50. g nacl and 2.5 l of water?
Answers
The concentration, in m/v percent, of a solution prepared from 50. g nacl and 2.5 l of water is 2 %.
The mass/volume percent is the ratio of a solution's total volume to the mass of the solute that makes up that solution. Since this type of concentration has been expressed as a percentage, the given proportion should be multiplied by 100.
The calculation if concentration is shown as:
It can be calculate as follows:
% NaCl = Mass of NaCl / Total mass × 100 %
% NaCl = 50 gram / 2500 × 100 %
% NaCl = 2 %.
As a result, the solution made from 50 g of nacl and 2.5 l of water will have a 2% concentration.
To know more about concentration
brainly.com/question/10695134
#SPJ4
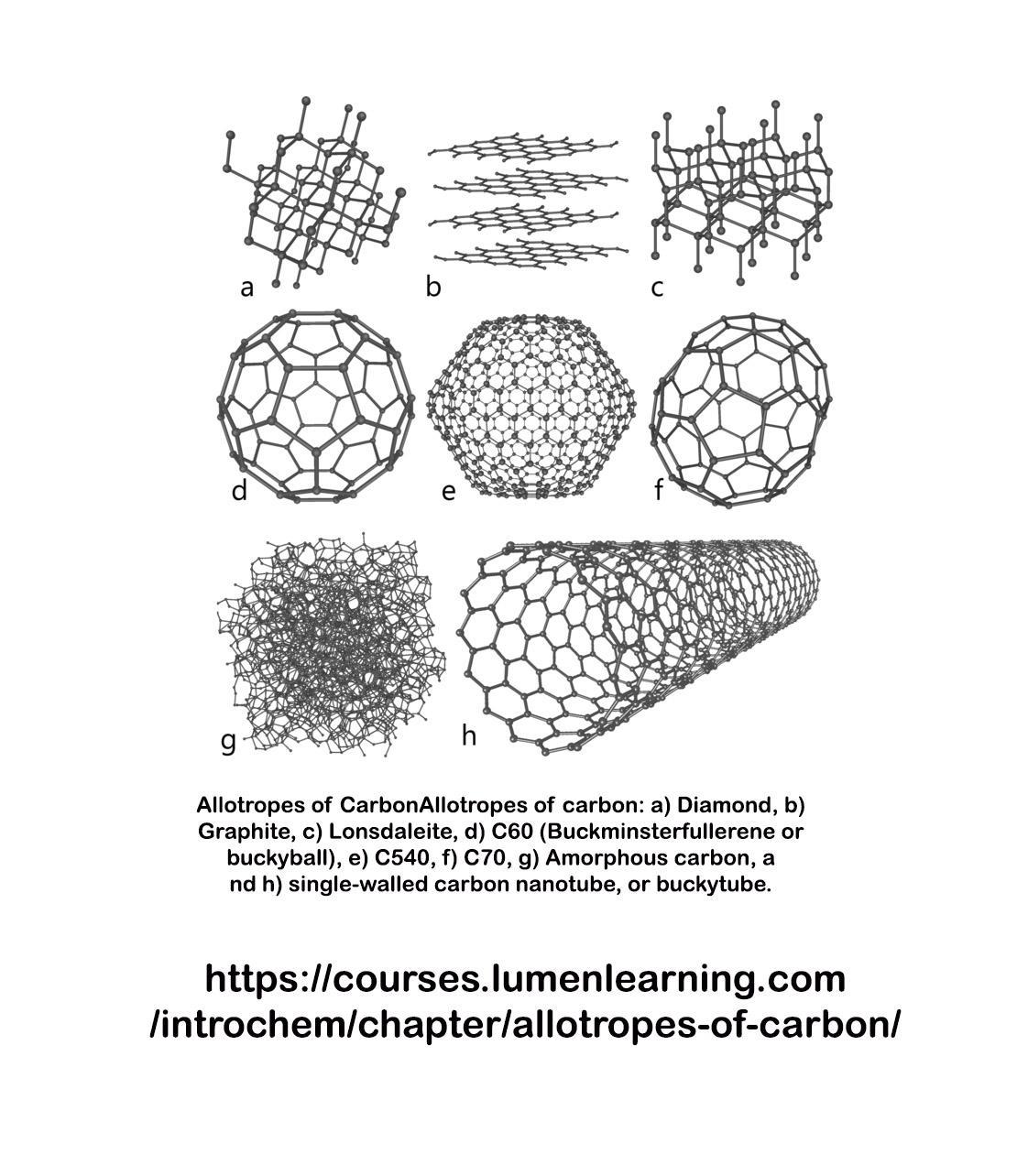
What name is given to the carbon-based structures used in lubricants and nanotubes
Answers
Answer:
Fullerene, also called buckminsterfullerene, any of a series of hollow carbon molecules that form either a closed cage (“buckyballs”) or a cylinder (carbon “nanotubes”).
Explanation:
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes.
Answer:
khansamueen has already provided an answer. This is an additional note on the many structures carbon may form, called allotropes.
Explanation: Carbon can take on many forms. They are all based only on the carbon atom, and are thus called alloptropes. One of these is diamond, "a" in the attached image.
Using a ph meter, you find the ph of an unknown solution to be 8. 0. How would you describe this solution?.
Answers
Answer:
Just Barely Base/Neutral
Explanation:
a pH of 8.0 is greater than Neutral (7.0) but is still neutral due to it being more neutral than a base
8. Which has greater thermal energy: a glass of water
or a lake full of water? Why?
Answers
Answer:
Im assuming a glass of water
Explanation:
there is less water that needs to be heated up
What’s the answer!?!!

Answers
Answer: generic material and protein coat. Have a great day
Explanation:
Classify: Heat that is transferred in a circular motion with warm particles rising and cool
particles sinking in a gas and liquid is called____
Convection
Conduction
Radiation
Answers
hello bro please set instar
Ionic Compounds
Recall, non-metals form
ions called
ions called
and metals form
Metals and non-metals bond because
the metals want to get rid of extra electrons and the non-metals want to gain extra
electrons so that both can have a full outer electron shell. For example:
Answers
Answer:
non metal forms anions
metals for cations
nonmetals and metals bond because of electron transfer
an example is NaCl(Sodium chloride)
Rosa is planning an investigation using a microscope to try to identify a group of cells. She sees that the cells are joined together, so she knows that they are from one organism. If she also sees that all of the cells have cells walls, Rosa can conclude that she could be looking at bacterial cells. human cells. mouse cells. plant cells.
Answers
Answer:
either bacterial cells or plant cells but I think bacterial
Explanation:
from google: Bacteria are single celled microbes. Bacteria are classified into five groups according to their basic shapes: spherical (cocci), rod (bacilli), spiral (spirilla), comma (vibrios) or corkscrew (spirochaetes). ... They can exist as single cells, in pairs, chains or clusters.
- cannot be human or mouse cells as both are animals and animal cells do not have cell walls
Which table shows a proportional relationship between x and y? Responses x 2 4 7 8 y 6 10 21 24x 2 4 7 8 y 6 10 21 24 , x 4 7 8 10 y 2 3. 5 4 5x 4 7 8 10 y 2 3. 5 4 5 , x 40 50 60 90 y 8 10 14 18x 40 50 60 90 y 8 10 14 18 , x 1 2 3 4 y 6 8 10 12x 1 2 3 4 y 6 8 10 12 ,
Answers
The table that shows a proportional relationship is given as follows:
x: 4 7 8 10
y: 2 3.5 4 5
Proportional Relationship:
A proportional relationship is a relationship between two variables with equal proportions. Another way to think about them is that in a proportional relationship, one variable is always a constant value multiplied by another variable. This constant is known as the "proportional constant".
Direct Proportional Relationship:
This type describes a direct relationship between two quantities. Simply put, when one value increases, the other value also increases and vice versa.
For example, when a car speeds up, it travels a longer distance in a given amount of time. In notation, a direct proportion is denoted by y ∝ x.
Inverse Proportional:
This type describes an indirect relationship between two quantities.
Simply put, when one value increases, the other value decreases and vice versa y ∝ 1/x. For example, by increasing the speed of a car, it can travel a fixed distance in less time.
Based on the Question:
For proportional ratios, the ratio between the output and input variables must be constant for all values in the table, so the correct option is given in the third table as
2/4 = 0.5.
3.5/7 = 0.5.
4/8 = 0.5.
5/10 = 0.5.
and the ratio equation: y = 0 and 5 times.
Learn more about Proportional Relationship:
https://brainly.com/question/29765554
#SPJ4
which of the following is a diatomic molecule: hydrogen (h2), aluminum (al), sulfur (s8), or carbon (c)?
Answers
The diatomic molecule is hydrogen (H2).
Diatomic molecules are composed of two atoms of the same element that are chemically bonded together. Aluminum (Al), sulfur (S8), and carbon (C) are not diatomic molecules as they exist as single atoms or in larger molecular structures.
To determine which of the following is a diatomic molecule: hydrogen (H2), aluminum (Al), sulfur (S8), or carbon (C), let's look at the chemical formulas.
A diatomic molecule consists of two atoms of the same element bonded together. Among the given options:
1. Hydrogen (H2) - has two hydrogen atoms bonded together.
2. Aluminum (Al) - is a single aluminum atom.
3. Sulfur (S8) - has eight sulfur atoms bonded together.
4. Carbon (C) - is a single carbon atom.
Considering these details, the diatomic molecule in this list is hydrogen (H2)..
Learn more about diatomic molecule
brainly.com/question/1433575
#SPJ11
The metal component that is protected from corrosion is called the?
a) Cathode
b) Anode
c) Rectifier
d) Electron
Answers
The metal component that is protected from corrosion is called the option A: cathode.
Metal surfaces experience corrosion, an electrochemical process, when they come into contact with electrolytes. Corrosion is the process of converting a metal back to its original form as an ore; during this transformation, the metal disintegrates and loses structural integrity. Pipelines, structures, and ships all make use of these metal surfaces.
It is crucial to make sure that these metals endure as long as possible, which calls for cathode protection. Cathode is a metal rod placed in an electrolyte where oxidation takes place so that it loses electrons in the electrolyte and get oxidized. Zinc metal is generally used as a cathode electrode.
To know more about corrosion, refer:
https://brainly.com/question/29854677
#SPJ4
Part 1: Predict which compound in each pair is more acidic. Explain your answers
. a) cyclopentanol or 3-clorophenol
b) cyclohexanol or cyclohexantiol
c) cyclohexanol or cyclohexanecarboxylic acid
d) 2,2-dichlorobutan-1-ol or butan-1-ol
Part 2: Predict which compound in each group is more soluble in water. Explain your answers.
a) butan-1-ol, pentan-1-ol or propan-2-ol
b) chlorocyclohexane, cyclohexanol or cyclohexane-1,2-diol
c) phenol, cyclohexanol or 4-methylcyclohexanol
Answers
(1a) 3-Chlorophenol is more acidic than cyclopentanol (chlorine atom electron-withdrawal). (1b) Cyclohexanecarboxylic acid is more acidic than cyclohexanol (stronger carboxylic acid group).
(2a) Propan-2-ol is more soluble in water than butan-1-ol and pentan-1-ol (hydrogen bonding ability). (2b) Cyclohexanol is more soluble in water than chlorocyclohexane (hydroxyl group enables hydrogen bonding).
Part 1: Comparing Acidic Strength
a) 3-chlorophenol is more acidic than cyclopentanol. This is because the presence of a chlorine atom in 3-chlorophenol can stabilize the negative charge on the phenoxide ion through inductive and resonance effects, making it more stable and easier to form.
b) Cyclohexanecarboxylic acid is more acidic than cyclohexanol. The carboxylic acid group (-COOH) is a stronger acid functional group compared to the hydroxyl group (-OH) present in cyclohexanol.
c) 2,2-dichlorobutan-1-ol is more acidic than butan-1-ol. The presence of the electron-withdrawing chlorine atoms in 2,2-dichlorobutan-1-ol enhances the acidity by stabilizing the negative charge on the alkoxide ion formed upon deprotonation.
d) Cyclohexanecarboxylic acid is more acidic than cyclohexanol. The carboxylic acid group (-COOH) is a stronger acid functional group compared to the hydroxyl group (-OH) present in cyclohexanol.
Part 2: Comparing Solubility in Water
a) Propan-2-ol is more soluble in water than butan-1-ol and pentan-1-ol. Propan-2-ol has a hydroxyl group (-OH) that can form hydrogen bonds with water molecules, increasing its solubility.
b) Cyclohexanol is more soluble in water than chlorocyclohexane. The presence of the hydroxyl group in cyclohexanol allows for hydrogen bonding with water molecules, enhancing its solubility. Chlorocyclohexane, on the other hand, is nonpolar and lacks the ability to form significant hydrogen bonds with water.
c) Cyclohexanol is more soluble in water than phenol and 4-methylcyclohexanol. Both cyclohexanol and phenol can form hydrogen bonds with water, but phenol's aromatic ring reduces its solubility. 4-methylcyclohexanol is also less soluble than cyclohexanol due to the steric hindrance from the methyl group, which disrupts hydrogen bonding.
To know more about the aromatic ring refer here,
https://brainly.com/question/32170261#
#SPJ11
Combustion analysis of fluorene, a polycyclic aromatic hydrocarbon used to make dyes, plastics, and pesticides, produces 11.44 g CO2 and 1.80 g H2O. Calculate the empirical formula for fluorene.
Answers
Combustion analysis of fluorene, a polycyclic aromatic hydrocarbon used to make dyes, plastics, and pesticides, produces 11.44 g CO2 and 1.80 g H2O. The empirical formula of fluorene C₆H₄.
CₓHₐ + O2 → CO2 + H2O Since the combustion is complete, so the total mass of the reactants is equal to the total mass of the reactants Therefore, Mass of O2 = (17.9 + 9.14) – 5.9 = 21.14 grams Here, the empirical formula for the hydrocarbon is CₓHₐ. So, now we have to need to determine the number of moles of the O2, CO2, and H2O :- Mole of CO2 = 17.9/44 = 0.4068 Mole of H2O = 9.14/18 = 0.5078 Mole of O2 = 21.14/32 = 0.6606. The empirical formula for the compound containing uranium and fluorine is UF6. Explanation: The empirical formula of a compound represents the lowest whole number ratio of elements in the compound. This ratio is represented by subscripts in the formula. by this information, we can consider that combustion analysis of fluorene, a polycyclic aromatic hydrocarbon used to make dyes, plastics, and pesticides, produces 11.44 g CO2 and 1.80 g H2O. The empirical formula of fluorene C₆H₄.
Learn more about Combustion:
brainly.com/question/14521417
#SPJ4
Find the density of 250 mL (volume) soy milk which has a mass of 276.3 grams. Use the density equation (mass ÷ volume).
Answers
Answer:
density =mass/volume
=276.3/250
=1.105g/ml
The following differential equation describes a chemical reaction,
dx
dy
=e
−y
(2x+1) where y is the amount of chemical product and x is the length across the reactor. i. Find the particular solution for y, given that y=0 at the edge of the reactor where x=0. [2 marks] ii. Use the particular solution in part i. to find the amount of chemical product, y, at a distance of x=1.
Answers
The amount of chemical product, y, at a distance of x = 1 is given by y = -ln(-(1/2) ln|3| + C3), where C3 is a constant.
The given differential equation is dx/dy = e^(-y)(2x+1), where y represents the amount of chemical product and x represents the length across the reactor.
i. To find the particular solution for y, we need to solve the given differential equation. Let's separate the variables and integrate both sides with respect to x and y.
dx/(2x+1) = e^(-y) dy
Integrating both sides, we get:
∫ dx/(2x+1) = ∫ e^(-y) dy
To integrate the left side, we can use the substitution u = 2x+1. This gives us du = 2dx, which implies dx = du/2.
∫ dx/(2x+1) = ∫ (1/u) (du/2)
= (1/2) ∫ du/u
= (1/2) ln|u| + C1, where C1 is the constant of integration.
= (1/2) ln|2x+1| + C1
Integrating the right side:
∫ e^(-y) dy = -e^(-y) + C2, where C2 is the constant of integration.
Now, equating both sides and simplifying:
(1/2) ln|2x+1| + C1 = -e^(-y) + C2
Rearranging the terms:
e^(-y) = -(1/2) ln|2x+1| + C3, where C3 = C2 - C1.
Taking the natural logarithm of both sides:
-y = ln(-(1/2) ln|2x+1| + C3)
y = -ln(-(1/2) ln|2x+1| + C3), where C3 is a constant.
ii. To find the amount of chemical product, y, at a distance of x = 1, we substitute x = 1 into the particular solution obtained in part i.
y = -ln(-(1/2) ln|2(1)+1| + C3)
Simplifying further:
y = -ln(-(1/2) ln|3| + C3)
Learn more about differential equations from the given link:
brainly.com/question/1164377
#SPJ11
why is it more effective to perform an extraction with several small portions of solvent as opposed to one large portion of solvent of equal volume? byu
Answers
It is more effective to perform an extraction with several small portions of solvent as opposed to one large portion of solvent of equal volume because the amount of the material left in the trash will be less.
The extraction of certain ratio of the solute is able to the distribute among the phases during each extraction. The various extractions with the lesser amounts of the solvent are more efficient than the single extraction with the huge amount of solvent.
The extraction is about to maximize the outside field of the communication between the two solvents, we can easily get the more surface area in the contact with the fewer amounts.
To learn more about extraction here
https://brainly.com/question/14522836
#SPJ4
Name one diatomic compound which is mononuclei
Answers
Answer:
Common diatomic molecules include hydrogen (H2), nitrogen (N2), oxygen (O2), and carbon monoxide (CO). Seven elements exist as homonuclear diatomic molecules at room temperature: H2, N2, O2, F2, Cl2, Br2, and I2. The bond in a homonuclear diatomic molecule is non-polar due to the electronegativity difference of zero.
What is the reaction ship between cells and tissues

Answers
Answer:
the answer is a bc tissues are a bunch of cells clumped together and tissues make up organs
Explanation:
4. After virtual school you decide to make a milkshake. You know that the best
milkshake is made with 500 ml of milk and 120 g of chocolate ice cream.
Chocolate ice cream weighs 0.6 g/ml. How many liters of ice cream should you
add?
120 ml
2401
O 0.21
4.0 di
Answers
Answer:
The answer actually isn't all that complicated. 120 grams is equivalent to 120 milliliters, and 120 milliliters is equal to 0.21 liters, since you divide 120 by 1000.
explain how is the electrochemical gradient used to import k and no3- (channel, cotransport)*
Answers
The electrochemical gradient is used to import K ions through selective potassium channels and NO₃⁻ ions through cotransport mechanisms, where the energy from the electrochemical gradient of another ion drives their import.
To explain how the electrochemical gradient is used to import potassium (K) and nitrate (NO₃⁻) ions through channels and cotransport mechanisms:
1. An electrochemical gradient is created by the differences in ion concentration and electrical potential between the inside and outside of a cell. This gradient provides the driving force for the movement of ions across the cell membrane.
2. To import K ions, cells use channels called potassium channels. These channels are selective for K ions and allow them to pass through the membrane down their electrochemical gradient, moving from an area of high concentration to an area of low concentration.
3. For NO₃⁻ import, cells use a cotransport mechanism. In this process, the electrochemical gradient of another ion, typically H⁺ or Na⁺, is used to drive the transport of NO₃⁻ into the cell. This occurs through a cotransporter protein, which binds both ions simultaneously.
4. When the cotransporter protein binds the driving ion (H⁺ or Na⁺) and NO₃⁻, the electrochemical gradient of the driving ion provides the energy required for the import of NO3- into the cell, even if it is against its own electrochemical gradient
To learn more about electrochemical gradient https://brainly.com/question/30033619
#SPJ11
what is the difference between thermal energy and heat ?
Answers
There is really no difference thermal energy and heat are pretty the same thing.
Answer:
Thermal energy refers to the energy contained within a system that is responsible for its temperature. Heat is the flow of thermal energy.
What is the reaction ship between cells and tissues

Answers
Answer:
A. Tissues are made up of cells
Explanation:
I just googled it :3
Which chemical equation is correctly balanced?
O Mg(NO3)2 + 2K2CO3 →→ MgCO3 + 2KNO3
O Mg(NO3)2 + K2CO3 → MgCO3 + 2 KNO3
2Mg(NO3)2 + K2CO3 - 2MgCO3 + KNO3
Mg(NO3)2 + K2CO3
MgCO3 + KNO3
Answers
Answer:
Mg (NO3)2+ K2CO3 ⇒ Mg CO3 + KNO3
Mg=1 ,N =2, O =6 +3 9 ,K =2 ,C=1 , (reactant side)
Mg =1, C = 1 ,O = 3+3 = 6 , K= 1., N =1 (product side)
Mg(NO3)2 + K2CO3 ⇒ Mg CO3 + 2KNO3
Mg =1 , C = 1, O =3+6=9, K = 2 IN product side
ANS:
Mg (NO3)2 +K2CO3 ⇒ MgCO3 +2 KNO3
Explanation:
The box in the above picture is falling from the top of a building to the ground. Two major forces are acting on the box as it falls. Which force is represented by the arrow labeled Q?
A.
the force of magnetism
B.
the force of gravity
C.
the net force of the object
D.
the force of air resistance

Answers
Answer:
I think it's B
Explanation:
apologies if I get this wrong
Answer:
The correct answer is B. The force of gravity.
Explanation:
Gravity affects all objects falling through the Earth's atmosphere. Gravity pulls down on a falling object.
( I got it on study Island as well )
How
many
molecules are in 8.55x10-15 moles of a substance
Answers
Answer:
Know this
1mole of a substance contains 6.022x10²³molecules
Now
8.55x10^-15moles x 6.022x10²³molecules/1mole
=5.15x10^9molecules.
Heat transfer is due to molecules colliding with neighboring molecules during _____, while heat transfer is due to molecules actually moving to another location during
Answers
Heat transfer which occurs when molecules collide with neighboring molecules is Conduction while heat transfer which is due to molecules actually moving to another location is Convection.
What is Heat transfer?This is the process in which thermal(heat) energy is moved from a hot region to a cold one.
There are three main types of heat transfer which are:
ConductionConvectionRadiationThe first two types is explained above and is the most appropriate choice.
Read more about Heat transfer here https://brainly.com/question/16055406
which form of energy does a cheeseburger have
Answers
Answer:
chemical potential energy - It mainly has chemical potential energy, this is really a type of electrical potential energy stored in the chemical bonds of the molecules
Explanation:
You can give the other person brainliest, I don't need it! :)