The chapter ends with gatsby telling nick part of the story of his brief relationship with daisy
five years earlier and nick's claim that you can't repeat the past... followed by gatsby's
rebuttal. we'll see how the last three chapters play out to see who is more correct. at this
point, who is more correct, why?
Answers
Nick is more correct because he understands that the past cannot be recreated exactly as it was. Gatsby is delusional and obsessed with an idealized version of Daisy that does not match reality. He thinks that he can erase the five years that Daisy spent with Tom and make her love him as she did before. He does not realize that Daisy has changed and that their relationship was based on his lies about his social status and wealth. He also ignores the fact that Daisy loves Tom in some way and that she enjoys the lifestyle that Tom provides for her. Gatsby's attempt to repeat the past is doomed to fail because he cannot change who Daisy is or what she wants.
About obsessedObsessed is a tendency to think or focus excessively on one thing or a persistent thought, thereby interfering with one's daily life and potentially interfering with one's mental health.
Learn More About Obsessed at https://brainly.com/question/29546238
#SPJ11
Related Questions
No links please and please help

Answers
Practice Run Just need a bit of help! Good amount of points!
According to LeChatelier's principle, what are 3 general ways that you can reverse a reaction when it is at equilibrium. Select all that apply.
Adding concentration of product
Adding concentration of product
Adding concentration of reactant
Adding concentration of reactant
Keeping the concentration the same
Keeping the concentration the same
Changing temperature
Changing temperature
Changing the state of matter
Changing the state of matter
Changing the color
Changing the color
Changing Pressure
Answers
The 3 general ways by which a system in equilibrium can be reversed are by changing the concentration of the reactants of products, changing the pressure of the system, and changing the temperature of the system.
Le Chatelier's principleLe Chatelier's principle state that when a reaction is in equilibrium and one of the constraints that affect the rate of reactions is applied to the system, the equilibrium shifts so as to cancel out the effects of the constraints.
The constraints being referred to by Le Chatelier are concentration, pressure, and temperature. Increasing or decreasing the pressure of a system in equilibrium will shift the equilibrium to the sides with the lower moles or higher moles respectively.
Increasing the concentration of the reactants will shift the equilibrium toward the product side while increasing the concentration of the products will shift the equilibrium toward the reactant side.
In the same vein, increasing the temperature of a system will sift the equilibrium towards the product if the system itself is endothermic. If the system is exothermic, a reversal will occur.
More on Le Chatelier's principle can be found here: https://brainly.com/question/2001993
#SPJ1
Describe the three main groups of clay minerals. Explain the
differences in their structure and stability?
Answers
The three main groups of clay minerals are kaolinite, smectite, and illite. Each group has a unique structure and stability, which affects their properties and behavior in various applications.
Kaolinite is a 1:1 clay mineral, meaning that it has one tetrahedral sheet of silica (SiO4) and one octahedral sheet of alumina (AlO6) stacked on top of each other. The layers are held together by hydrogen bonding and van der Waals forces. Kaolinite is relatively stable and has a low cation exchange capacity (CEC), which means that it has a low ability to adsorb and exchange cations. Kaolinite is commonly used in ceramics, paper, and paint industries.
Smectite is a 2:1 clay mineral, meaning that it has two tetrahedral sheets of silica and one octahedral sheet of alumina stacked on top of each other. The layers are held together by strong electrostatic forces and water molecules in the interlayer space. Smectite has a high CEC and can adsorb and exchange cations, which makes it useful in various applications, such as drilling fluids, catalysts, and soil amendments. Smectite is also known for its swelling properties,
when ethane burns in air to form carbon dioxide and water ,heat energy is released. Explain why energy is released
Answers
Energy is released because it is a combustion reaction.
Combustion reaction -
Hydrocarbon bonds are broken during combustion events, and more energy is always released during the formation of water and carbon dioxide bonds than was consumed to break the initial hydrocarbon bonds. Burning materials that are primarily made of hydrocarbons produces energy because of this. This is an exothermic reaction.
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O + Heat
(ethane)
In the process of burning, or combustion, the reactant takes up oxygen and oxidizes it, releasing energy in the form of heat and light. It happens quickly. Along with the energy created during burning, carbon dioxide and water vapor are also produced.
Hence heat and light which are forms of energy are released during combustion reaction.
To learn more about Combustion reaction refer- https://brainly.com/question/13251946
#SPJ9
which diagram represents an electrolytic solution
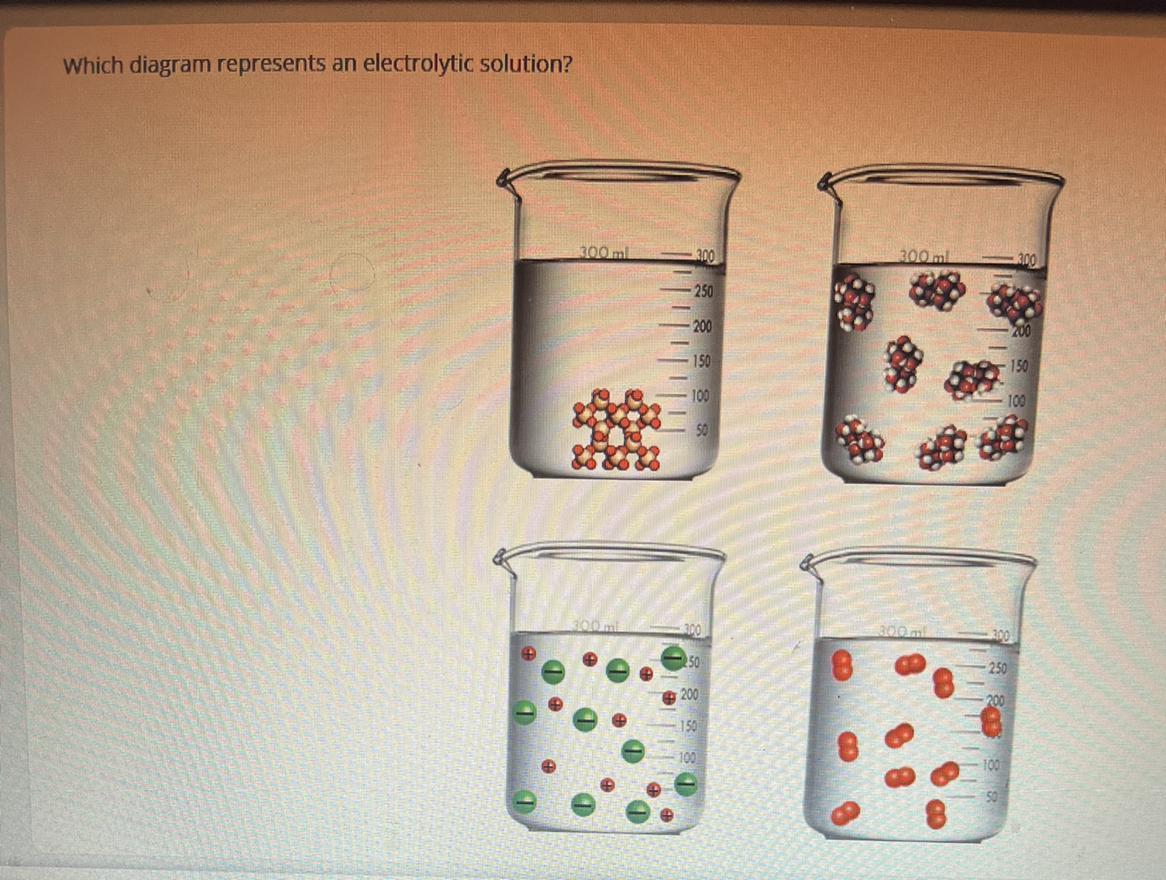
Answers
Answer:
Image
Explanation:
An electrolytic solution is a solution that has the ability of conducting electricity. Electrolytic solutions contain ions in which it conducts electricity from those ions. Ions are charged atoms. This solution (refer to image) has ions, as represented by the + and - symbols.

A car starts from rest and reaches a top speed of 80 m/s. If the car did this is 20 seconds . What is the acceleration ?
Answers
somethingExplanation:
a 24.4−g sample of ethylene glycol, a car radiator coolant, loses 752 j of heat. what was the initial temperature of the ethylene glycol if the final temperature is 32.5°c? (c of ethylene glycol
Answers
The initial temperature of the ethylene glycol was approximately 19.77°C.
To solve this problem, we can use the formula for heat transfer:
q = mcΔT
Where:
q = heat transferred (in joules)
m = mass of the substance (in grams)
c = specific heat capacity of the substance (in J/g°C)
ΔT = change in temperature (in °C)
We are given:
mass of ethylene glycol = 24.4 g
heat transferred = 752 J
final temperature = 32.5°C
We need to find the initial temperature, so let's rearrange the formula:
q = mcΔT --> ΔT = q / (mc)
Substituting the given values:
ΔT = 752 J / (24.4 g * c)
Now, we can solve for c by using the specific heat capacity of ethylene glycol, which is usually around 2.42 J/g°C.
ΔT = 32.5°C - initial temperature
Plug in the values:
32.5°C - initial temperature = 752 J / (24.4 g * 2.42 J/g°C)
Simplify the equation:
32.5°C - initial temperature = 752 J / 59.168 g
Now, solve for the initial temperature by isolating it:
initial temperature = 32.5°C - (752 J / 59.168 g)
Calculating the value:
initial temperature = 32.5°C - 12.73°C
Therefore, the initial temperature of the ethylene glycol was approximately 19.77°C.
Know more about initial temperature:
https://brainly.com/question/2264209
#SPJ11
The equilibrium constant, Kc, for the reaction H2 (g) + 2 (g) 2H (g) is 54 at 425 ºC. If the equilibrium mixture contains 0.030 M H and 0.015 M 2 , the equilibrium concentration of H2 is______.
Answers
Answer:
[H₂] = 0.0011M
Explanation:
Based on the chemical equation:
H₂(g) + I₂(g) ⇄ 2HI(g)
And Kc is defined as:
Kc = [HI]² / [H₂] [I₂]
Where Kc = 54
And [] represents equilibrium concentration of each compund.
Replacing:
54 = [0.030M]² / [H₂] [0.015]
[H₂] = [0.030M]² / 54* [0.015]
[H₂] = 0.0011Mwhy is the mass of kcl recovered less than the starting mass of khco3
Answers
The mass of KCl recovered can be less than the starting mass of KHCO3 due to several factors, such as:
1. Incomplete conversion: The reaction between KHCO3 and HCl to form KCl involves a stoichiometric ratio. If the reaction is not driven to completion or if there are side reactions or competing reactions, it may result in an incomplete conversion of KHCO3 to KCl. This would lead to a lower mass of KCl recovered compared to the starting mass of KHCO3.
2. Losses during the process: During the reaction and subsequent processes like filtration or drying, some of the product (KCl) or reactant (KHCO3) may be lost. Losses can occur due to physical losses like splattering or spilling, or chemical losses like volatilization of certain compounds.
3. Impurities or contaminants: The starting KHCO3 may contain impurities or contaminants that do not convert to KCl during the reaction. These impurities or contaminants can remain in the reaction mixture or be lost during subsequent purification steps, leading to a difference in the mass of KCl recovered.
It is important to ensure proper reaction conditions, efficient conversion, and minimize losses during handling and purification to achieve a higher recovery of the desired product.
To know more about mass of KCl refer here
https://brainly.com/question/17489670#
#SPJ11
what are the signs of the enthalpic and entropic terms for formation of secondary structure motifs (e.g., α-helices or β-sheets)?
Answers
The enthalpic term is favorable, while the entropic term is unfavorable for the formation of secondary structure motifs.
Are the enthalpic and entropic terms favorable for secondary structure motif formation?In the context of secondary structure motifs like α-helices or β-sheets, the enthalpic term refers to the energy changes associated with the formation of hydrogen bonds and other stabilizing interactions within the motif.
The enthalpic term is generally favorable for the formation of secondary structures since the establishment of these interactions contributes to the stability and structural integrity of the motif.
On the other hand, the entropic term relates to the changes in molecular freedom or disorder upon the formation of secondary structure motifs.
When a protein adopts a specific secondary structure, there is a reduction in conformational flexibility, resulting in a decrease in entropy.
This entropic term is typically unfavorable for the formation of secondary structures since it restricts the range of accessible conformations for the protein.
Overall, the enthalpic term, driven by favorable interactions, promotes the formation of secondary structure motifs, while the entropic term, driven by reduced conformational flexibility, poses an unfavorable contribution to the process.
Learn more about enthalpic term
brainly.com/question/30516012
#SPJ11
What is the compound name of BaSO4?
What is the molar mass?
What is the mass in grams of 2.3 mols of the compound
Answers
The inorganic substance with the chemical formula BaSO4 is barium sulfate (or sulphate). It is a tasteless, crystalline white substance that is insoluble in water.
BaSO4 stands for barium sulfate.A barium cation and a sulfate anion are the two elements that make up barium sulfate. There are four oxygen atoms joined to the sulfur. A sulfate salt of barium, known as BaSO4, is present in the mineral barite. It is a white crystalline substance that is soluble in strong acids but insoluble in water and alcohol.
Is barium the same as bromine?Elements include both barium and bromine. Barium is a metal belonging to Group 2 of the modern periodic table, and bromine is a non-metal belonging to Group 17 (halogen).
To know more about chemical formula visit:-
https://brainly.com/question/29031056
#SPJ1
The best option for a donor organ would be _____.
Answers
Answer:
Most likely a heart or lung
Explanation:
a lot of people have a lot of heart problems and hearts are the most vital organ in your body. without your heart you could not live and your heart has a tissue tear it could be lethal causing death
I need some help with with question:
How could you prepare 100 ml of a 0.40 mol/L of MgSO4 solution from a stock solution of 2.0 mol/L MgSO4. And then how would u prepare it?
Answers
Answer:
C1V1=C2V2
C1 is 2.0mol/l
V1=?
C2=.4mol/L
V2=100ml or for this 0.1L
V1 is 20ml
Best way to prepare this is to measure out 20ml of the 2 molar solution and add 80mL to it to get to 100mL
Explanation:
Mechanism of cell destruction of Poison oak
Answers
Poison oak (Toxicodendron diversilobum) and its eastern counterpart poison ivy (T. radicans) are two of the most notoriously painful plants in North America. The mechanism is similar to poison oak because it causes the body's immune system to destroy cancerous skin cells.
the third law of thermodynamics describes the entropy of a: select the correct answer below: solid liquid gas all of the above
Answers
The third law of thermodynamics describes the entropy of a: solid.
The third law of thermodynamics states that the entropy of a pure crystalline substance approaches zero as the temperature approaches absolute zero (0 Kelvin or -273.15 degrees Celsius). This law implies that at absolute zero, a perfectly ordered and pure crystalline solid will have zero entropy.
The third law of thermodynamics is not specific to liquids or gases but applies to solids. In a solid, the molecules are highly ordered and have fixed positions in a regular lattice structure. As the temperature decreases towards absolute zero, the thermal motion of the molecules reduces, and the system becomes more ordered, resulting in a decrease in entropy.
In contrast, liquids and gases have higher entropy compared to solids at absolute zero because their molecules have more freedom of movement and are not as tightly arranged. Therefore, the third law of thermodynamics specifically addresses the entropy of solids and does not apply to liquids or gases.
To learn more about law of thermodynamics, here
https://brainly.com/question/1368306
#SPJ4
A 35 L tank of oxygen is at 42°C with an internal pressure of 5000.mmHg. If the temperature changes to 88°C, what would the new pressure be ? the volume is held constant
Answers
Answer:
5730 mmHg.
Explanation:
The following data were obtained from the question:
Initial temperature (T1) = 42 °C.
Initial pressure (P1) = 5000 mmHg.
Final temperature (T2) = 88 °C.
Final pressure (P2) =.?
Next we shall convert celsius temperature,T(°C) to Kelvin temperature, T(K).
This can be obtained as follow:
T(K) = T(°C) + 273
Initial temperature (T1) = 42 °C.
Initial temperature (T1) = 42 °C + 273 = 315 K
Final temperature (T2) = 88 °C.
Final temperature (T2) = 88 °C + 273 = 361 K
Finally, we shall determine the new pressure.
Since the volume of the container is constant, the new pressure can be obtained as follow:
Initial temperature (T1) = 315 K.
Initial pressure (P1) = 5000 mmHg.
Final temperature (T2) = 361 K.
Final pressure (P2) =.?
P1/T1 = P2/T2
5000/315 = P2/361
Cross multiply
315 x P2 = 5000 x 361
Divide both side by 315
P2 = (5000 x 361) / 315
P2 = 5730.1 ≈ 5730 mmHg
Therefore, the new pressure is 5730 mmHg.
A soap solution with a volume of 25.0 ml was titrated to the endpoint with 0.5 M HCL. If 15.0ml of the acid was used, what was the molarity of the sop solution (assume a 1:1 mol ration)?
Answers
The molarity of the soap solution used in the titration reaction is 0.003 M
How do i determine the molarity of the soap solution?The molarity of the soap solution can be obtain as shown below:
Volume of soap solution (Vs) = 25.0 mLMolarity of acid (Ma) = 0.5 MVolume of acid (Va) = 15.0 mLMole ratio = 1Molarity of soap solution (Ms) = ?MaVa / MsVs = Mole ratio
(0.5 × 15) / (Ms × 25) = 1
0.075 / (Ms × 25) = 1
Cross multiply
Ms × 25 = 0.075
Divide both side by 25
M = 0.075 / 25
Ms = 0.003 M
Thus, we can conclude that the molarity of the soap solution is 0.003 M
Learn more about molarity:
https://brainly.com/question/13386686
#SPJ1
What is one way that potential energy might be involved in rescue team missions?
Answers
Answer:
You may be saving someone from a rock slide, where the rocks will fall at any time, before they fall they have potential energy.
The one way that potential energy might be involved in rescue team missions to save someone to fall is potential energy is used to hold one.
What is gravity?Gravity is the interaction that is because of the force of energy between the surface of the earth to the mass or any energy present on the earth's surface and the force becomes double with the weight.
The potential energy is a form of energy that is used against gravity to do the work which required energy and in rescue, the energy is needed in every coss to save one.
Therefore, one way that potential energy might be involved in rescue team missions to save someone to fall is because gravitational potential energy is used to hold one.
Learn more about gravity, here:
https://brainly.com/question/13810323
#SPJ2
Please help me and I’ll give you brainiest!!!!!!!!!
Have a wonderful day!!:)))

Answers
Answer:
Explanation:
C
What is the role of Cl2 in the following
reaction?
CL + NaOH Nacl+ NaCO3+H2O
Answers
Answer:
Cl2 acts as an oxidizing agent
Explanation:
The balanced equation is
Cl2+NaOH⟶NaCl+NaClO3+H2O
Cl2 acts as a reactant in this chemical reaction
Cl2 receives one electron and thus reduces itself from Cl2 to 2 Cl−. This indicates that 2Cl- will oxidize some other element and hence Cl2 act as an oxidizing agent.
Question 8
I need help

Answers
Answer:A
Explanation: Since the boiling point is 212. As salt keeps getting added the boiling temperature keeps going up. Meaning it will boil at a high temp.
Assume that 1. 5 g of lauric acid is combusted and all heat energy released is transferred to a 325 g sample at 25 degrees C. Calculate the final temperature if delta Hcombustion is -37 kJ/G and the specific heat of water is 4. 18 J/gK
Answers
88 degrees the final temperature if delta Hcombustion is -37 kJ/G and the specific heat of water is 4. 18 J/gK
delta H= Cs* delta T
delat T= delta h / cs(specific heat)
delta T= -37/4.18
delta T = 88 degrees
A calorimeter is used to calculate combustion heats. The specific heat capacity of a substance is defined as the amount of energy needed to raise one gramme of that substance's temperature by one degree Celsius. A material will store heat energy more effectively if its specific heat capacity is higher. When one mole of a substance burns, the quantity of energy produced as heat (q) is measured as the heat of combustion (H°c) (combustion). When a reaction generates heat, it is an exothermic process that releases energy.
Learn more about temperature here:
https://brainly.com/question/11464844
#SPJ4
what is the mass of electron
Answers
Explanation:
The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 1/1,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom.
Answer: The Mass of an electron is 9.1093837 x 10^-31 kgs
Explanation:
Arrange the gases in order of decreasing density when they are all under STP conditions.
Neon , Helium, Florine, Oxygen
Answers
The correct order of decreasing density of the gases under STP conditions is Fluorine, Oxygen, Neon, and Helium.
Under STP conditions, the gases can be arranged in order of decreasing density as follows:
Fluorine > Oxygen > Neon > Helium
Fluorine has the highest density due to its relatively heavy atomic mass compared to the other gases. Oxygen follows next with a density higher than Neon and Helium. Neon has a density higher than Helium as it has a larger atomic size and atomic mass than Helium. Helium, being the lightest gas, has the lowest density among the given gases under STP conditions.
The higher the molar mass, the greater the density of the gas. Here are the molar masses of the given gases:
1. Helium (He) - 4 g/mol
2. Neon (Ne) - 20 g/mol
3. Fluorine (F2) - 38 g/mol
4. Oxygen (O2) - 32 g/mol
Using the molar masses, we can arrange the gases in decreasing order of density:
1. Fluorine (F2)
2. Oxygen (O2)
3. Neon (Ne)
4. Helium (He)
Under STP conditions, the gases in decreasing order of density are: Fluorine, Oxygen, Neon, and Helium.
Learn more about STP conditions here:-
https://brainly.com/question/31609879
#SPJ11
The equation for photon energy, E, is E=hcλ where h = 6.626×10−34 J⋅s (Planck's constant) and c = 2.99×108 m/s (the speed of light). What is the wavelength, λ, of a photon that has an energy of E = 4.61×10−19 J ?
Answers
Answer:
9.133×10^-6 m
Explanation:
A photon is the smallest discrete amount or quantum of electromagnetic radiation. It is the basic unit of all light. Photons are always in motion and, in a vacuum, travel at a constant speed to all observers of 3 x 10^8 m/s.
From the question, the following details were supplied;
Energy of the photon (E) = 4.61×10^−19 J
Speed of light (c) = 2.99×10^8 m/s
Plank's constant (h) = 6.626×10^−34 J⋅s
Wavelength of the photon (λ)= ??? The unknown
From
E=hc/λ
λ= hc/E
Substituting values
λ= 6.626×10^−34 × 2.99×10^8/ 4.61×10^−19
λ= 91.33×10^-7 m
λ= 9.133×10^-6 m
If a 600 gram radioactive sample goes through 3 half-lives, how would be left?
Answers
Answer:
75 g
Explanation:
1 half life = 600/2 = 300g
2 half life = 300/2 = 150g
3 half life = 150/2 = 75g
The amount of 600 gram remaining after 3 half-lives is 75 g.
The half life of a substance is given by:
\(N=N_o(\frac{1}{2} )^\frac{t}{t_\frac{1}{2} } \\\\where\ N\ is\ substance\ remaining\ after\ t\ years,N_o\ is\ the \ original\ sample\\t_\frac{1}{2}\ is\ the\ half\ life\\\\\\Given\ that:\\N_o=600\ g, t=3t_\frac{1}{2} , hence:\\\\N=600(\frac{1}{2} )^\frac{3t_\frac{1}{2} }{t_\frac{1}{2} } \\\\N=600(\frac{1}{2} )^3=75\ g\)
The amount of 600 gram remaining after 3 half-lives is 75 g.
Find out more at: brainly.com/question/4483570
Below is a statement of assertion followed by a statement of reason. Choose the correct answer out of the following choices. Assertion: Sodium alginate is insoluble in water Reason: Ionic salts are soluble in water Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion. Both assertion and reason are wrong statements. Assertion is correct, but reason is wrong statement. Both assertion and reason are correct statements, and reason is the correct explanation of the assertion Assertion is wrong but reason is correct statement
Answers
Assertion is correct, but reason is wrong statement(d) is a statement of assertion followed by a statement of reason.
The assertion that sodium alginate is insoluble in water is correct. However, the reason given that ionic salts are soluble in water is not a correct explanation.
While most ionic salts are indeed soluble in water, there are exceptions such as insoluble ionic salts that do not dissolve in water. Sodium alginate is one such exception. It is a salt of alginic acid, which is a polysaccharide, and the sodium ions are not easily dissociated in water. Hence, sodium alginate is insoluble in water.
For more questions like Assertion click the link below:
https://brainly.com/question/30443467
#SPJ11
The method adopted in the separation of lead (II) Chloride from water is
Answers
Answer:
distillation
Explanation:
Try to dissolve the solid mix in boiling water. Silver chloride will not dissolve, we can filtrate to isolate solid silver chloride. In the filtrate is a solution of lead(II) chloride and sodium chloride. Let this solution cool and lead chloride will precipitate, we can again filtrate to isolate lead chloride.
So definitely, it is distillation.
What is the product of a reaction between a strong acid and a sulfite ion (which acts as a base)?
Answers
\(SO_{2}\) is the product of a reaction between a strong acid and a sulfite ion (which acts as a base).
A chemical reaction in which an acid and a base interact quantitatively is known as neutralisation or neutralisation. By neutralising a reaction in water, surplus hydrogen or hydroxide ions are removed from the solution.
Despite being a weak base, the sulfite ion can hydrolyze to form basic solutions. The equilibria in an acidic solution are changed to create sulphurous acid, which leads to the development of SO2 gas. A colourless gas with a distinctive suffocating smell, sulphur dioxide.
The reaction mechanism is as follows;
\(SO_{3}^{2-} (aq) + H_{2} O(l)\) ⇒ \(HSO_{3} ^{-} (aq) + OH^{-} (aq)\)
\(HSO_{3} ^{-} (aq) + H_{2} O(l)\) ⇒ \(H_{2} SO_{3} (aq) + OH^{-} (aq)\)
\(H_{2} SO_{3} (aq)\) ⇒ \(H_{2} O (l) + SO_{2} (g)\)
Therefore, \(SO_{2}\) is the product of a reaction between a strong acid and a sulfite ion (which acts as a base).
Learn more about neutralization reaction here:
https://brainly.com/question/15095136
#SPJ4
a diatomic molecule contains i. atoms of two different elements bonded together with a covalent bond. ii. two atoms of the same element bonded together with a covalent bond. iii. two lone pairs of electrons.
Answers
A diatomic molecule consists of two atoms of the same element or two atoms of different elements, bonded together with a covalent bond, or in some cases, two lone pairs of electrons. So all statements are true.
A diatomic molecule is a molecule made up of two atoms of the same element or two atoms of different elements bonded together. The bonding of the atoms is usually done through a covalent bond, meaning that electrons are shared between the two atoms in order to create a stable arrangement. In the case of two atoms of different elements, each atom has a different electronegativity, resulting in the formation of a polar covalent bond. This means that the electrons will be pulled closer to one atom than the other, resulting in an overall dipole moment for the molecule. In the case of two atoms of the same element, a nonpolar covalent bond is formed. This means that the electrons are shared equally between the two atoms and no dipole moment is formed. In some cases, two lone pairs of electrons may be present instead of a covalent bond. This results in a molecule with a larger overall dipole moment.
Learn more about diatomic molecule: https://brainly.com/question/30595473
#SPJ11