the synthesis of aspirin is a common esterification reaction. it is based on the balanced reaction given in the background of this lab. calculate the theoretical yield of aspirin given that 3.52-ml of acetic anhydride was reacted with 0.9457-g of salicylic acid.
Answers
The theoretical yield of aspirin is 1.23 g
Aspirin is a common drug with many uses. It relieves pain and reduces the risk of serious problems such as heart attack and stroke. It comes in many forms, including:
It is important to remember that aspirin alone does not lower blood pressure. However, its ability to thin the blood may benefit some people with high blood pressure.
moles of salicylic acid = mass / molar mass
=> 0.9457 / 138.2 = 0.006843 mol
Acetic anhydride is in excess. Thus salicylic acid is the limiting reactant.
Total moles of aspirin that can form = moles of salicylic acid = 0.006843
mass of aspirin = number of moles of aspirin x molar mass of aspirin
= 0.006843 mol x 180.16g/mol = 1.23g
Thus theoretical yield of aspirin = 1.23 g
Learn more about aspirin here:-https://brainly.com/question/13533428
#SPJ4
Related Questions
A coin collector has a bag of 50 pennies. What is weighted average of a penny in the sample according to the data?

Answers
The weighted average of a penny is = 67.9g
Calculation of average weightThe sample of penny for pre 1982 is a total of = 18 pennies.
The mass of each penny for pre 1982 = 3.1
Therefore the total weight for penny for pre 1982
= 18×3.1
= 55.8
The sample of penny for post 1982 is a total of = 32 pennies.
The mass of each penny for post 1982 = 2.5
Therefore the total weight for penny for post 1982
=32×2.5
= 80
The weighted average
= 55.8+80/2
= 135.8/2
= 67.9g
learn more about mass here:
https://brainly.com/question/14156113
#SPJ1
to a first approximation the ionization constant of h2s is
Answers
The ionization constant of H₂S is approximately 1.0 x 10⁻⁷.
The ionization constant, also known as the acid dissociation constant (Ka), is a measure of the extent to which an acid dissociates in water. It indicates the degree of ionization of an acid and is typically expressed as the equilibrium constant for the reaction between the acid and water.
In the case of H₂S (hydrogen sulfide), it is a weak acid that can partially dissociate in water to produce hydrogen ions (H⁺) and sulfide ions (HS⁻). The ionization reaction can be represented as follows:
H₂S ⇌ H⁺ + HS⁻
The ionization constant (Ka) represents the equilibrium expression for this reaction. The value of Ka determines the relative strength of the acid. For H₂S, the ionization constant is approximately 1.0 x 10⁻⁷, indicating that it is a weak acid.
This value indicates that H₂S only partially ionizes in water, with a small fraction of H₂S molecules dissociating into H⁺ and HS⁻ ions. The majority of H₂S remains in its molecular form.
It is important to note that the ionization constant can vary depending on factors such as temperature and concentration. The given approximation is a typical value at standard conditions.
To know more about "Ionization constant" refer here:
https://brainly.com/question/30639622#
#SPJ11
What is the third quantum number of a 3s² electron in phosphorus,
1s²2s²2p 3s²3p³?
A. m₁ = 3
B. m₁ = 2
C. m₁ = 0
D. m, = 1
Answers
Answer:
The third quantum number of a 3s² electron in phosphorus is m₁ = 0. The electron configuration of phosphorus is [Ne]3s²3p³. The outermost shell is the third shell, so here, n = 3.
I hope this helps!
Explanation:
(´▽`ʃ♡ƪ)
In an effort to sanitize his water, Beethoven knows that bromine can be used to purify water. If Beethoven has 2.12 x 1022 formula units of aluminum bromide are reacted with excess chlorine gas, how many milliliters of liquid bromine (density = 3.12 g/mL) are formed?
Answers
Answer:
2.7 mL
Explanation:
The equation of the reaction is;
2AlBr3 + 3Cl2 -----> 2AlCl3 + 3Br2
Number of moles in 2.12 x 1022 formula units of aluminum bromide
1 mole of AlBr3 = 6.02 * 10^23 formula units
x moles = 2.12 x 1022 formula units
x = 2.12 x 1022 formula units * 1 mole/ 6.02 * 10^23 formula units
x = 0.0352 moles of AlBr3
According to the reaction equation;
2 moles of AlBr3 produces 3 moles of Br2
0.0352 moles of AlBr3 produces 0.0352 moles * 3 moles /2 moles
= 0.0528 moles of Br2
Mass of Br2 produced = 0.0528 moles of Br2 * 159.808 g/mol
Mass of Br2 produced = 8.44g
But density = mass/volume
volume = mass/density
volume of Br2 = 8.44 g/ 3.12 g/mL
volume of Br2 = 2.7 mL
Which transition by an electron will release the greatest
amount of energy?
6 0
n = 4
n = 3
Ο Α
A
n = 2
оооо
n = 1
D
+
B
D
Next
Submit
Mark this and return
Answers
Answer:
A.A
Explanation:
I took the quiz and I got that answer
79. Which best describes what is made of matter? (1 point)
all living things and objects
all atoms
all solid objects and atoms
all living things
Answers
Answer:
all living things
Explanation:
Students were asked to select methods to increase the rate of dissolving a solid. Which methods would increase the rate? Select all that may apply.
Select 3 correct answer(s)
1. Increasing the pressure so that a solid dissolves in the solution.
2. Shaking/stirring the mixture causing an increase in the rate of dissolution.
3. Grinding the solute to increase the surface area.
4. Decreasing the pressure so that a solid dissolves in the solution.
5. Increasing the temperature in order to increase molecule collisions.
Answers
5. Increasing the temperature to increase molecule collisions.
What factor increases the solubility of a solid in a solution?An increase in the temperature of the solution can increase the solubility of a solid solute. For example, a greater amount of sugar will dissolve in warm water as compared to in cold water. The size of solute particles, stirring of the solution and increasing temperature of the solution are the three factors that can affect the solubility of a solid in a solvent. Increasing the surface area of the solute will also increase the rate of dissolving in a solution as well as increase the temperature of the solvent. Stirring will increase the speed which also increases the rate of dissolving a solid solute in a solution which helps in attaining higher solubility.
So we can conclude that temperature is the factor that increases the solubility of a solid into a solution.
Learn more about solubility here: https://brainly.com/question/23946616
#SPJ1
Answer:
Increase temp
Shaking/ stirring
Grinding
Explanation:
Chatgpt hehehehe
The resistance of a thermometer is 5 ohm at 25 degree Celsius and 6 2 at 50 degree Celsius. Using linear approximation, calculate the value of resistance temperature coefficient at 45 degree Celsius.
Answers
The approximate resistance value at 45 degrees Celsius is around 5.8 ohms.
To calculate the value of the resistance temperature coefficient at 45 degrees Celsius using linear approximation, we can use the formula:
R(T) = R0 + α(T - T0),
where R(T) is the resistance at temperature T, R0 is the resistance at a reference temperature T0, α is the resistance temperature coefficient, and (T - T0) is the temperature difference.
Given that the resistance at 25 degrees Celsius is 5 ohms (R0 = 5) and the resistance at 50 degrees Celsius is 6 ohms (R(T) = 6), we can calculate the value of α.
6 = 5 + α(50 - 25),
Simplifying the equation:
1 = 25α,
Therefore, α = 1/25 = 0.04 ohm/degree Celsius.
Using the linear approximation, we can approximate the value of the resistance at 45 degrees Celsius:
R(45) = 5 + 0.04(45 - 25) = 5 + 0.04(20) = 5 + 0.8 = 5.8 ohms.
Therefore, the value of the resistance at 45 degrees Celsius is approximately 5.8 ohms.
You can learn more about resistance at
https://brainly.com/question/17563681
#SPJ11
How do you write balance equations Fe2 S3+O2-Fe2O3+SO2
Answers
Answer:
2Fe2 S3+6O2 → 2Fe2O3+3SO2
Explanation:
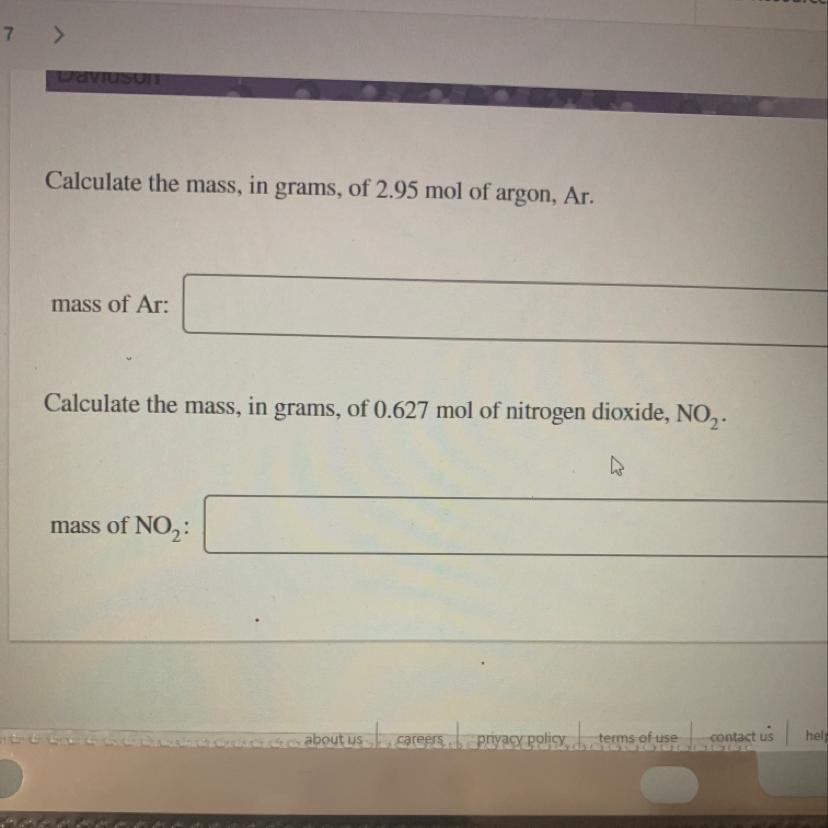
Calculate the mass, in grams, of 2.95 mol of argon, Ar.
mass of Ar:
Calculate the mass, in grams, of 0.627 mol of nitrogen dioxide, NOZ.
mass of NO2:
Answers
Answer:
118 g of Ar; 28.8 g of \(NO_{2}\)
Explanation:
To convert from moles to grams, you simply multiply by the atomic mass. So the conversion table looks as follows:
\(\frac{2.95 mol}{1} *\frac{39.95 g}{1 mol}\)
The mol units cancel, and you are left with 118 g of Argon (117.8525 without sig-figs).
As for the next part of our problem, the first thing we need to do is find the molar mass of \(NO_{2}\). e can do this by adding the weight of all the atoms within the compound:
N + 2(O) = (14.01) + 2(16) = 46.01 g
Then, we repeat the above process, except we use the molar mass we just found instead of the atomic weight:
\(\frac{0.627 mol}{1}*\frac{46.01 g}{1 mol}\)
Once again, the mol units cancel, and we are left with 28.8 g of \(NO_{2}\) (28.84827 before sig-figs).
Is NaOH a strong base?
Answers
Yes, NaOH, is a strong base.
NaOH, also known as sodium hydroxide
In aqueous solutions, it fully dissociates to form hydroxide ions (OH-). Strong bases have a high degree of dissociation, meaning they break apart completely in water to form their respective ions.
In contrast, weak bases only partially dissociate in water. Strong bases have a pH greater than 7, and NaOH has a pH of around 13.
Strong bases are also highly alkaline and can cause chemical burns if handled improperly. They are commonly used in industrial applications such as soap making, paper production, and oil refining.
A strong base is a substance that ionizes completely or almost completely in an aqueous solution. It releases hydroxide ions (OH-), which increases the hydroxide ion concentration and raises the pH of the solution. Strong bases have a high degree of dissociation, meaning they break apart completely in water to form their respective ions.
To learn more about strong base refer here
https://brainly.com/question/16749233
#SPJ11
what is the mass of electron
Answers
Explanation:
The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 1/1,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom.
Answer: The Mass of an electron is 9.1093837 x 10^-31 kgs
Explanation:
NO LINKS PLS HELP
Which weighs more a sealed, half-filled jar of water or that same jar after it is placed in the freezer until the water turns to ice? How do you know the answer without experimenting?
Answers
Answer:
frozen
Explanation:
I would say because when u freeze water it expands and denifys.
a 22.4 l container at 0 oc contains 0.30 mol n2, 0.20 mol o2, 0.40 mol he and 0.10 mol co2.what is the partial pressure (in atm) of oxygen?
Answers
The partial pressure of oxygen is 0.49 atm.
To find the partial pressure of oxygen, we need to first calculate the total pressure of the mixture using the ideal gas law:
PV = nRT
where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin.
Rearranging this equation, we get:
P = nRT/V
Substituting the given values, we have:
P = (0.30 + 0.20 + 0.40 + 0.10) mol x 0.0821 L·atm/mol·K x 273 K / 22.4 L
= 2.44 atm
The partial pressure of oxygen can be calculated by multiplying the mole fraction of oxygen by the total pressure:
moles of O2 = 0.20 mol
moles of all gases = 0.30 + 0.20 + 0.40 + 0.10 = 1.00 mol
mole fraction of O2 = moles of O2 / moles of all gases = 0.20 / 1.00 = 0.20
partial pressure of O2 = mole fraction of O2 x total pressure = 0.20 x 2.44 atm = 0.49 atm
Therefore, the partial pressure of oxygen is 0.49 atm.
To know more about partial pressure, refer here:
https://brainly.com/question/31214700#
#SPJ11
Calculate the frequency of yellow light with a wavelength of 580 x 10–9 m.
Answers
Answer:
5791
Explanation:
Easy peasy lemon squeazy
The basic unit of electric current is the:
ohm.
ampere.
* conductivity.
volt.
Answer is ampere
Answers
Answer:
It's B
Explanation:
Edge2021
PLS I NEED HELP ITS IMPORTANT I AM GIVING 25 POINTS HELP PLEASE. I NEED IT FAST
Why is it important for cells to be able to turn genes on and off? Select ALL Correct reasons!
Question 2 options:
A. so the cell is not too chaotic
B. so the cell can respond to the environment
C. so the cells can grow uncontrollably
D.so the cell does not waste energy
Answers
Answer:
B
Explanation: Since you need this quick, its because it needs to respond to the environment. I dont remember the exact term
4. Which of the following is not an example of a disturbance that might jumpstart
secondary succession?
a. An ecosystem being destroyed by pollution.
b. A long period of little or no rainfall resulting in a drought.
C. A hurricane causing a devastating flood.
d. A volcano forming a new island in a chain.
Answers
Answer: D: a volcano forming a new island in a chain
Explanation:
explain your observations in terms of lechatelier's principle. how come we have to use 12 m hcl here? how would the equilibrium shift if we used 1 m hcl instead?
Answers
The Le Chatelier principle explains how an equilibrium changes when its conditions change. For variations in concentrations, temperature, or pressure, the shift's direction can be predicted. Although catalysts speed up the process of reactions reaching equilibrium, they do not change the location of an equilibrium.
The equilibrium will change to create more products if the concentration of the reactants (quantity of reactants) rises (product-favored). The reaction will change to produce additional reactants as the number of products rises (reactant-favored). Reactants benefit from a decrease in reactants. Products benefit from decreasing product. A system may gain temperature from the environment or as a result of a chemical interaction. The equilibrium moves to the right as the temperature drops (products). In other words, the system favors the reaction that produces heat to make up for the drop in temperature.
Learn more about Le Chatelier principle here:
https://brainly.com/question/29501115
#SPJ4
How many grams are in 2.04 x 10^24 molecules of H2O
Answers
Answer: 0.611 grams in 2.04 x 10^24 molecules of H2O
Explanation:
To find out how many grams are in 2.04 x 10^24 molecules of H2O, we need to use the molar mass of water.
The molar mass of water is 18.015 g/mol.
To convert the number of molecules to the number of moles, we multiply by Avogadro's number, which is 6.022 x 10^23 molecules/mol.
2.04 x 10^24 molecules x 1mol/6.022 x 10^23 molecules = 0.034 mol
Then we multiply this number of moles by the molar mass of water to find the number of grams:
0.034 mol x 18.015g/mol = 0.611g
Therefore, there are 0.611 grams in 2.04 x 10^24 molecules of H2O.
PLEASE HELP ME ASAP
An ion has 16 protons, 17
neutrons, and 18 electrons. What
is the correct isotope notation?
A. As-2 B. CI-1
33
17
C. 335-2 D. 32S-2
16
16
Enter the answer choice letter.

Answers
Answer: d
Explanation:
12.
Contrast and compare:
a) ionic and covalent bonding
Answers
Answer:
Ionic bonds - an intramolecular force exist in a nonmetal and metal compounds such as NaCl. The Na donate 1 electon to Cl to complete its octet rule.
Covalent bond - an intramolecular force exist in a nonmetal and nonmetal compounds such as bonds O2, Cl2, CO2, sugar, proteins and most of organic compounds and biomolecules by sharing electrons to bond.
There are two types of covalent bonds: polar and nonpolar. Polar bond is a bond between two different nonmetal atoms of different electronegativities. While nonpolar bond is a bond between the same atom or two differenct atoms of the same electronegativities (if there is). Their electronegativities pull will cancel so that their overall polarity is zero.
The Michaelis-Menten equation describes the rate of an enzyme-catalyzed reaction as a function of the concentration of the _____
Answers
The Michaelis-Menten equation describes the rate of an enzyme-catalyzed reaction as a function of the concentration of the substrate. This equation is derived from the Michaelis-Menten kinetics, which is a fundamental model for enzyme kinetics.
The Michaelis-Menten equation is expressed as follows:
V = (Vmax * [S]) / (Km + [S])
Where:
- V represents the reaction rate or velocity of the enzyme-catalyzed reaction.
- Vmax is the maximum velocity or rate of the reaction when the enzyme is saturated with the substrate.
- [S] represents the concentration of the substrate.
- Km is the Michaelis constant, which is a measure of the affinity of the enzyme for the substrate.
The Michaelis-Menten equation illustrates that at low substrate concentrations, the reaction rate increases linearly with increasing substrate concentration. However, as the substrate concentration continues to rise, the rate approaches a maximum (Vmax) due to the saturation of the enzyme's active sites.
Km, on the other hand, represents the substrate concentration at which the reaction rate is half of the maximum. It reflects the affinity of the enzyme for the substrate, with lower Km values indicating higher affinity.
In summary, the Michaelis-Menten equation describes how the rate of an enzyme-catalyzed reaction depends on the concentration of the substrate, providing insights into the kinetics and efficiency of enzyme-substrate interactions.
Learn more about enzymes at https://brainly.com/question/1596855
#SPJ11
A decrease of pH by 3 implies A) The H* concentration triples. B) The OH- concentration decreases by a factor of 3. C) The OH- concentration decreases by a factor of 1000. D) The H* concentration decreases by a factor of 1000.
Answers
A decrease of pH by 3 implies :C) The OH- concentration decreases by a factor of 1000.
What is the relationship between pH and OH?pH is a scale used to determine the hydroxide ion (OH–) concentration in a solution. It is equal to the negative log of hydrogen ion (H+) concentration and is also equal to the negative log of hydroxide ion (OH– ) concentration. As the pH of a solution increases by one pH unit, concentration of OH- increases by ten times.
When the value of pOH is less than 7, then it is considered basic and therefore there are more OH- than H+ in the solution. At pH 7, the substance is at neutral and means that the concentration of H+ and OH- ion is same. If pH < 7, then solution is acidic.
To know more about pH, refer
https://brainly.com/question/172153
#SPJ4
atoms of nonmetallic elements form covalent bonds, but they can also form ionic bonds. how is this possible? group of answer choices this happens when one of the bonded nonmetallic elements has a strong electronegativity. an ionic bond results when a nonmetallic elements loses an electron to a metallic element. it happens when one of the nonmetallic elements loses an electron to become a positive ion. an ionic bond results when a nonmetallic elements gains an electron from a metallic element.
Answers
Atoms of nonmetallic elements form covalent bonds, but they can also form ionic bonds. An ionic bond results when a nonmetallic elements gains electrons to form an ion.
Hydrogen, carbon, nitrogen, oxygen, phosphorus, sulphur, silicon, boron, tellurium, and selenium are among the non-metallic elements in the periodic table. They also consist of noble gases and halogens (such as fluorine, chlorine, bromine, iodine, and astatine) (helium, neon, argon, krypton, xenon and radon).
Nonmetals are a group of seventeen elements, the majority of which are gases (hydrogen, helium, nitrogen, oxygen, fluorine, neon, chlorine, argon, krypton, xenon, and radon), one of which is a liquid (bromine), and a few of which are solids.
Hydrogen, carbon, nitrogen, oxygen, phosphorus, sulphur, silicon, boron, tellurium, and selenium are among the non-metallic elements in the periodic table. They also consist of noble gases and halogens (such as fluorine, chlorine, bromine, iodine, and astatine) (helium, neon, argon, krypton, xenon and radon).
Learn more about nonmetallic:
https://brainly.com/question/12621082
#SPJ4
According to Dalton's Law of Partial Pressures, the pressure of oxygen in dry air would be

Answers
The pressure of the oxygen in the air is 0.21 atm. The partial pressure of a gas is the contribution that gas makes to the total pressure when the gas is part of a mixture.
round 5,239 to 2 significant figures
Answers
Answer:
5240
Explanation:
I'm not sure tho sorry
You have decided that you want to make ice cubes. You put them in the freezer and they become solid at 32°F. What
temperature will the ice cubes melt?
32°F
100°F
OF
It's impossible to tell.
Answers
lago claro todo esta lleno de basura
Answer:
I think its about the same tempurature so i would say 32 degrees
Explanation:
Notice that the curve shows periods where the temperature does not change. These plateaus occur because energy is being used to changing the substance's phase, not raise the temperature. This is why water will not get hotter as it is boiling.
Another form of acid rain is one based around a nitrogen-containing acid. What acid might this be? What gases will react with water to produce this acid? Write balanced equations to demonstrate this.
Answers
Another form of acid rain is one based around a nitrogen-containing acid, which is nitric acid (HNO₃). Nitrogen dioxide (NO₂) and nitrogen monoxide (NO) gases will react with water to produce this acid.
What is acid rain?
Here are the balanced equations to demonstrate this:
1. Nitrogen monoxide reacts with oxygen to form nitrogen dioxide:
2NO + O₂ → 2NO₂
2. Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide:
2NO₂ + H₂O → HNO₃ + NO
These reactions lead to the formation of nitric acid, which contributes to acid rain.
To know more about Acid rain:
https://brainly.com/question/11543614?
#SPJ11
how many kilograms are in a 50 pound luggage bag?