Thermometer
Fractionating
Colu
Found-bottom
Mask
Bunsen burner
Water out
Condenser
Water in
set-up A
4
8
set-up B
c) Label the apparatus above as 'reflux' or 'distillation'.
d) Briefly explain the purpose of using a reflux condenser in an organic synthesis.
Answers
Answer:
a) Thermometer
b) Fractionating Column
c) Found-bottom Flask
d) Mask
e) Bunsen Burner
f) Water Out
g) Condenser
h) Water In
Set-up A: Reflux Set-up
Set-up B: Distillation Set-up
d) The purpose of using a reflux condenser in organic synthesis is to prevent the loss of volatile reactants or products. During a reflux reaction, the reactants are continuously heated, and the vapors are condensed and returned to the reaction vessel, which allows the reaction to proceed for an extended period without losing any material to the atmosphere. The reflux condenser also helps to maintain a constant temperature and prevent overheating.
(please could you kindly mark my answer as brainliest)
Related Questions
What is Decomposition Reaction
Answers
Answer:
Explanation:
Decomposition reaction, also known as analysis or dissociation, is a type of chemical reaction in which a compound breaks down into simpler substances or elements. In this reaction, a single reactant undergoes a chemical change and produces two or more products.
The decomposition reaction can be represented by the general equation:
AB → A + B
Where AB is the reactant, and A and B are the products. The reactant AB is usually a compound, and it breaks down into its constituent elements or simpler compounds.
There are different types of decomposition reactions, including:
Thermal decomposition: It occurs when a compound is heated, resulting in its decomposition into simpler substances. For example, the thermal decomposition of calcium carbonate (CaCO3) produces calcium oxide (CaO) and carbon dioxide (CO2):
CaCO3 → CaO + CO2
Electrolytic decomposition: It takes place when an electric current is passed through an electrolyte, causing it to break down into its component ions. For instance, the electrolysis of water (H2O) leads to the decomposition into hydrogen gas (H2) and oxygen gas (O2):
2H2O → 2H2 + O2
Photochemical decomposition: It occurs when a compound undergoes decomposition due to exposure to light energy. Chlorine gas (Cl2) can decompose into chlorine atoms (Cl) under the influence of light:
Cl2 → 2Cl
These are just a few examples of decomposition reactions. They are important in various chemical processes and are used in industries, laboratory experiments, and natural phenomena. By understanding and controlling decomposition reactions, scientists can gain insights into the behavior of different compounds and develop practical applications in fields such as chemistry, materials science, and environmental science.
Answer:
Explanation:
reaction in which a compound breaks down into simpler substances or elements
1. The author says that bog bodies were discovered as long ago as the 1600s, but the only ones existing today are those found after the late 1800s. What hap- pened to the earlier bog bodies?
Answers
Answer:
The earlier bog bodies that were discovered in the 1600s might have not been preserved properly due to a lack of knowledge on how to preserve them or a lack of awareness of their significance. It is also possible that they might have decayed and decomposed over time and not survived till the present day. However, the bog bodies found after the late 1800s were preserved and studied extensively due to the increasing awareness and understanding of their historical and archaeological significance.
Explanation:
Hope this helped!! Have a great day/night!!
how long does the non-oxidative energy system typically provide energy? group of answer choices 5 to 60 minutes 3 to 5 minutes 10 to 120 seconds up to 3 seconds
Answers
Anaerobic or non-oxidative energy system. The non-oxidative power system does not need oxygen to produce ATP, as the name suggests.
What does the word "anaerobic" mean in medicine?
Anaerobic is defined in medicine. When intense activity, anaerobic respiration takes place, pyruvic acid serves as a proton donor, and anaerobic bacteria cause lactic acid to build up in the tissues. 1a: alive, active, or happening in the lack of free oxygen
What advantages do anaerobic exercises have?
All people should engage in aerobic exercise to improve their overall health, strengthen their muscles, and reduce their risk of inflammation. Make sure you get off to a sluggish start. Increase the frequency, and duration gradually to give your body time to adjust to the new pace and avoid becoming hurt.
To know more about anaerobic visit:
https://brainly.com/question/11451338
#SPJ1
Outline the laboratory procedure for preparing a specific volume of a dilute solution from a concentrated stock solution
Answers
The various steps of how to prepare a specific volume of a dilute solution from a concentrated stock solution is described below.
Preparation of a dilute solution from stock solutionA stock solution is the solution that contains the standard concentration of a chemical compound.
A diluted solution is the solution shoes concentration has been decrease through addition of a solvent like water.
To prepare a dilute solution from a stock solution the following is done:
the needed volume of the stock solution is calculated,the volume is added to a volumetric flask,water is then added into the volumetric flask till it reaches the calibration line.Learn more about stock solutions here:
https://brainly.com/question/24697661
how many moles are in 100.0 g of gold?
Answers
Answer:
196.96655
Explanation:
The answer is 196.96655. I assume you are converting between grams Gold and mole. The molecular formula for Gold is Au. The SI base unit for the amount of substance is the mole.
The quantity present in an atom or molecules of a given substance is called moles it is written as mol and is calculated with the help of molar mass and mass of the given substance.
0.507699 mol is present in the 100 gm of gold.
Moles of gold can be calculated by:Given,
Mass (m) = 100 gmThe atomic weight of Au (M) = 196.967The formula for calculating moles is:
\(\rm Moles (n) = \dfrac{Mass}{Molar \;mass}\)
Substituting values in the formula:
\(\begin{aligned}\rm n & = \dfrac{100}{196.967}\\\\ & = 0.507699 \;\rm mol\\\\& = 0.5077\;\rm mol\end{aligned}\)
Therefore, 0.5077 mol is present in 100 gm of gold.
Learn more about moles here:
https://brainly.com/question/6260819
Why is the condensation of water vapor considered to be a process which hads up the air? a. Water yapar must nbsorb energy in order to condense. b. Air cain hold thore water in the liquld phase that the vapor phase. c. Energy is released by water vapor as it condenses. d. Liquid water has a lower specific heat than water vapor. QUESTION 60 a. 42% b. 2+5% c 90% d. 3376
Answers
The correct answer to the first part of your question is option (c): Energy is released by water vapor as it condenses.
When water vapor condenses into liquid water, it undergoes a phase change from a gaseous state to a liquid state. During this phase change, energy is released in the form of latent heat. This release of energy occurs because the water molecules in the vapor phase are more energetic and have higher kinetic energy compared to the water molecules in the liquid phase.
To know more about latent heat
brainly.com/question/23976436
#SPJ11
what is the daily carbohydrate intake range (in grams) that corresponds to a 50-60arbohydrate, 2000 calorie diet?
Answers
As a result, the daily carbohydrate intake range is 250 to 300 grams, which is equal to a diet with 50 to 60 grams of carbohydrates per 2000 calories.
What is carbohydrate?Carbohydrates can be found in a wide range of meals, both healthy and unhealthy, including bread, beans, milk, popcorn, potatoes, cookies, spaghetti, soft drinks, maize, and cherry pie. They are also capable of changing shapes. The most common and abundant types are sugars, fibers, and starches. A carbohydrate is a biomolecule with the empirical formula C in organic chemistry that consists of carbon, hydrogen, and oxygen atoms, typically in a hydrogen-oxygen atom ratio of 2:1.
What are the functions of carbohydrates and its types?Carbohydrates, or simply "carbs," are necessary at every stage of life. They act as the body's main source of energy and the preferred energy source for the brain. Carbohydrates are transformed by the body into glucose, a type of sugar. The cells, tissues, and organs of your body use glucose as fuel.
The three types of carbohydrates that are present in meals and beverages are starches, sugars, and fiber. On a food's nutritional information label, the term "total carbohydrates" refers to a combination of all three categories.
To know more about Carbohydrates visit:
https://brainly.com/question/11095186
#SPJ4
A solution is prepared by dissolving 23. 7 g of cacl2 in 375 g of water. The density of the resulting solution is 1. 05 g/ml. The concentration of cacl2 is __________% by mass.
Answers
A solution is prepared by dissolving 23. 7 g of cacl2 in 375 g of water. The density of the resulting solution is 1. 05 g/ml. The concentration of cacl2 is 0.5623 % by mass.
What is density?
How closely a material is packed together is determined by its density. The mass per unit volume is how it is formally defined. Symbol for density: D or Formula for Density: Where is the density, m is the object's mass, and V is its volume, the equation is: = m/V.
The density of a substance is a measurement of how tightly or loosely it is confined within a specific volume. We can travel through low-density materials like air, which has a density significantly lower than that of human tissue. Granite is a different material altogether. Never attempt to traverse stone.
Therefore, A solution is prepared by dissolving 23. 7 g of cacl2 in 375 g of water. The density of the resulting solution is 1. 05 g/ml. The concentration of cacl2 is 0.5623 % by mass.
To learn more about density
Here: https://brainly.com/question/1354972
#SPJ4
A hypothesis is best written as ?
Answers
Answer:
A good experimental hypothesis can be written as an if, then statement to establish cause and effect on the variables. If you make a change to the independent variable, then the dependent variable will respond
3. Predict the change in electronegativity of the next elements in a row (C, Si), then check those properties. Do they match your predictions?
Answers
The elements which come after 'C' and 'Si' are 'Ge' and 'Sn'. The element 'F' is the most electronegative element. Down the group the electronegativity generally decreases.
What is electronegativity?The electronegativity of an atom is defined as the relative tendency or power of the bonded atom in a molecule to attract the shared pair of electrons towards itself.
In general the electronegativity decreases on moving down a group. The increase in the size of atoms and the shielding effect of inner electrons decreases the electronegativity.
Here 'Ge' and 'Sn' comes below 'C' and 'Si'.
Thus the electronegativity of the elements after 'C' and 'Si' decreases.
To know more about electronegativity, visit;
https://brainly.com/question/14481608
#SPJ1
Give the reactions of orthoboric acid with :
- Sodium Hydroxide
- Ethyl Alcohol
Answers
Orthoboric acid is a weak acid with the chemical formula H3BO3. It can react with various compounds to form different products.
What are the reactions of orthoboric acid?Here are the reactions of orthoboric acid with sodium hydroxide and ethyl alcohol:
Reaction with Sodium Hydroxide:
When orthoboric acid reacts with sodium hydroxide (NaOH), it forms sodium borate and water. The balanced chemical equation for the reaction is:
H3BO3 + NaOH → Na[B(OH)4] + H2O
In this reaction, one molecule of orthoboric acid reacts with one molecule of sodium hydroxide to produce one molecule of sodium borate and one molecule of water.
Reaction with Ethyl Alcohol:
Orthoboric acid can react with ethyl alcohol (C2H5OH) to form triethyl borate and water. The balanced chemical equation for the reaction is:
3C2H5OH + B(OH)3 → B(O-C2H5)3 + 3H2O
In this reaction, three molecules of ethyl alcohol react with one molecule of orthoboric acid to produce one molecule of triethyl borate and three molecules of water.
Learn more about Orthoboric acid here: https://brainly.com/question/28321965
#SPJ1
pls help me with this chem question
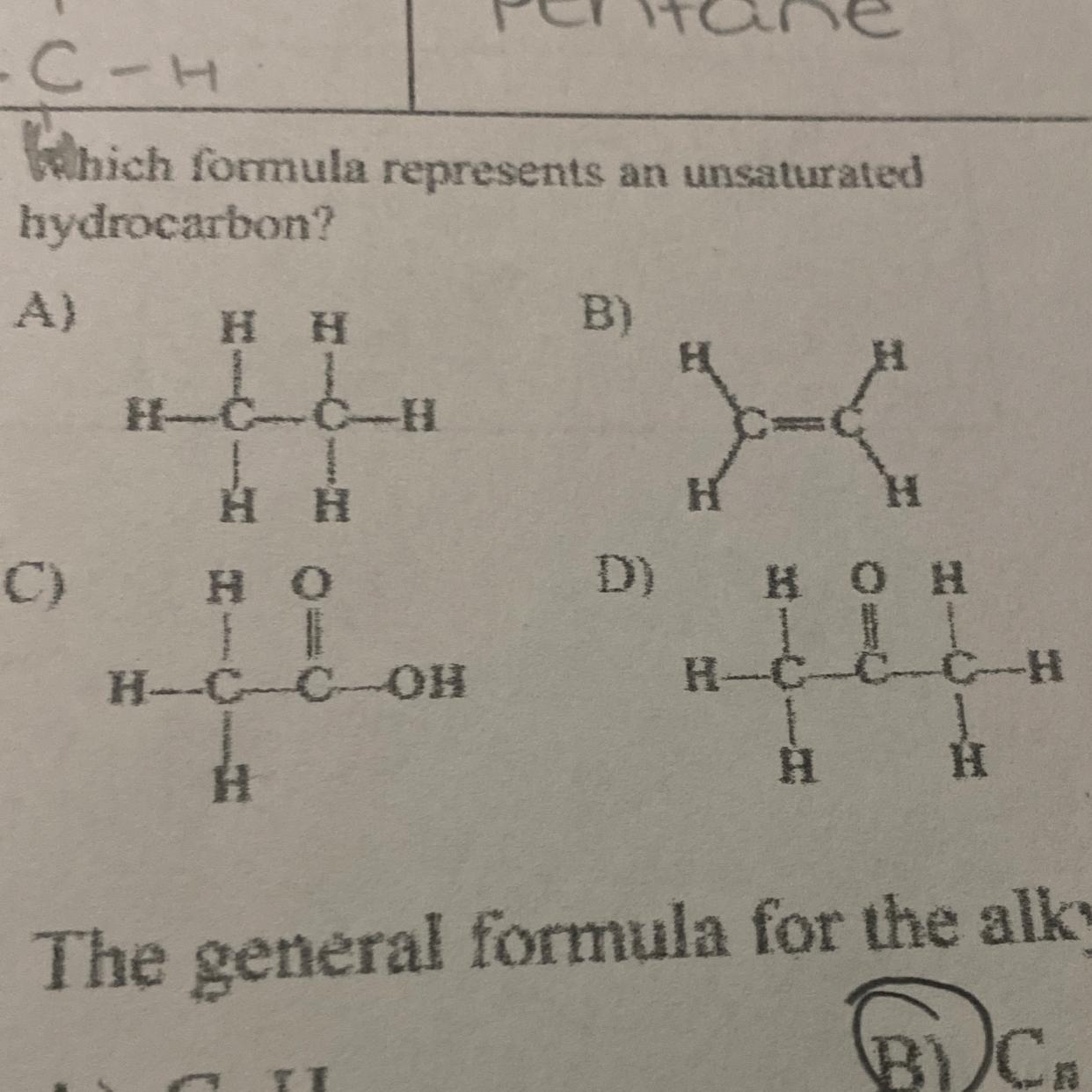
Answers
Answer:
B
Explanation:
i just know
Answer:
B
Explanation:
Hydrocarbons are with hydrogen and carbon only, so therefore you can immediately eliminate C and D. Therefore, we are left with A and B. Unsaturated hydrocarbons have double bonds or more, so B would be our answer (Double bond between carbon 1 and 2)
a(n) _________ is made up of two or more atoms held together by chemical bonds in a stable association. the atoms may or may not belong to the same element
Answers
A molecule is made up of two or more atoms held together by chemical bonds in a stable association. The atoms may or may not belong to the same element
Molecules refer to groups of two or more atoms that are held together by chemical bonds in a stable association. This bond is formed when the electrons that orbit the atoms' nucleus intermingle. As a result, the atoms' outermost shells fill up with electrons, providing stability to the molecule. The atoms in a molecule may belong to the same element or to different elements.
When they belong to different elements, the molecule formed is called a compound molecule. For example, carbon dioxide (CO₂) is a compound molecule that consists of one carbon atom and two oxygen atoms. The bond between these atoms is formed because the carbon atom requires two more electrons to fill its outer shell while the oxygen atoms require two electrons each to fill their outer shells, resulting in a stable molecule.
Therefore, a molecule is made up of two or more atoms held together by chemical bonds in a stable association. The atoms may or may not belong to the same element.
You can learn more about chemical bonds at: brainly.com/question/33297440
#SPJ11
What are the 4 main types of tissues in the human body?
Answers
Answer: nervous tissue connective tissue epithlial tissue muscle tissue
Explanation:
What is the formula Trisilicon tetra nitride
Answers
Tri = 3 -> 3 silicon atoms
Tetra = 4 -> 4 nitrogen atoms
Carbon cycle – What are the main reservoirs
of the carbon cycle? Where do the inorganic and organic carbon
cycles interact? What are the major differences and similarities
between the inorganic and organic carbon?
Answers
The main reservoirs of the carbon cycle are the atmosphere, oceans, land (including vegetation and soils), and fossil fuels. In these reservoirs, carbon exists in both inorganic and organic forms.
The inorganic carbon cycle involves the exchange of carbon dioxide (CO2) between the atmosphere and oceans through processes like photosynthesis and respiration.
Organic carbon, on the other hand, is found in living organisms, dead organic matter, and soil organic matter. It is cycled through processes such as decomposition and consumption by organisms. The interactions between the inorganic and organic carbon cycles occur primarily in the biosphere, where photosynthesis converts inorganic carbon into organic carbon compounds. While inorganic carbon is primarily in the form of CO2, organic carbon is present in complex organic molecules. Both forms of carbon play crucial roles in energy transfer, nutrient cycling, and climate regulation.
Learn more about Carbon Cycle
brainly.com/question/13729951
#SPJ11
Atoms of an element, X, have the electronic configuration shown below.
The compound most likely formed with magnesium, Mg, is:
(A) MgX
(B) Mg2X
(C) MgX2
(D) Mg3X2
Answers
The compound most likely formed with magnesium, Mg, is MgX . The correct option is (A) MgX.
The electronic configuration of the element, X is 2,8,7. Since X is located in group 7 of the periodic table, it has 7 electrons in its valence shell. In order to obtain a stable configuration, the atom can either gain one electron to fill the 3rd energy level completely or lose seven electrons to completely empty the 2nd energy level, which is easier. The resulting ion, X-, would have a stable electronic configuration of 2,8. Hence, the compound most likely formed with magnesium, Mg, is MgX .The correct option is (A) MgX.
Explanation: Magnesium is located in group 2 of the periodic table and has two valence electrons in its outermost shell. It loses these two electrons to form Mg2+ ions with stable electronic configurations of 2,8. On the other hand, element X gains an electron to form X- ions with stable electronic configurations of 2,8.The combination of Mg2+ and X- ions results in the formation of the compound MgX with a neutral charge.
Learn more about compound from:
https://brainly.com/question/14782984
#SPJ11
If you start with 50 grams of H2O how much NaOH would you produce
2 Na + 2 H2O = 2 NaOH + H2
Answers
Separate the redox reaction into its component half‑reactions. 3O2+4Co⟶2Co2O3 Use the symbol e− for an electron.
How would these be split into thier respective half-reactions?
Answers
The balanced half-reactions are: Oxidation half-reaction: 4Co → 4Co2+ + 8e-Reduction half-reaction: O2 + 2e- → 2O2 -
Given equation: 3O2+4Co⟶2Co2O3Using the symbol e− for an electron. The redox reaction can be broken down into two half-reactions. The reduction half-reaction is the one in which a species gains electrons and the oxidation half-reaction is the one in which a species loses electrons. The half-reactions are as follows: Reduction Half-reaction Half-reaction equation: O2 + 2e- → 2O2 -Oxidation number of oxygen in O2=0 and in O2^-= -1Charge on the left side = 0Charge on the right side = 2 x (-1) = -2Thus, 2 electrons are added to the left side to balance the charge, making the half-reaction:O2 + 2e- → 2O2 -Oxidation Half-reaction Half-reaction equation: 4Co → 4Co2+ + 8e-Oxidation number of cobalt in Co=0 and in Co2+ =+2Charge on the left side = 0Charge on the right side = 4 x (+2) + 8 x (-1) = -4Thus, 8 electrons are added to the right side to balance the charge, making the half-reaction:4Co → 4Co2+ + 8e-Thus, the balanced half-reactions are: Oxidation half-reaction: 4Co → 4Co2+ + 8e-Reduction half-reaction: O2 + 2e- → 2O2 -
learn more about reactions
https://brainly.com/question/32322541
#SPJ11
A pH strip was used to test the pH of a glass of water. The image shows the results.
Use the scale below to determine the pH value of the water, and determine whether the water is acidic, alkaline, or
neutral. Then predict what will happen to the pH if someone were to place a straw into the water and blow.

Answers
The pH strip is used to test the pH of a solution. The pH of water is neutral which is around 7.
What is pH?The pH is known as the power of hydrogen. The pH is used to measure the degree of basicity and acidity of a solution. The amount of hydrogen ion concentration in a solution determines the pH of the solution. Mathematically, pH is given by the formula:
pH -= -log [H⁺]
The pH strip is a strip of litmus paper with which a person can measure the pH value of a liquid solution. The substance in the pH paper causes the paper to show a different color at different acidity values. The official pH scale is between the pH values of 0 to 14, where 0 is very acidic and 14 very alkaline and 7 is neutral pH.
Learn more about pH here:
https://brainly.com/question/15289714
#SPJ1
Answer:
The pH value of the water is 7. And I don't exactly know what would happen if you put a straw into it and blew into it, but if I had to make a guess then I would guess that the pH value would go down because the water is moving around.
In the Haber Process, Ammonia is created. The balanced reaction is as follows: 3H2 + N2 --> 2NH3 What type of reaction is this?
Decomposition
Single Replacement
Synthesis
Double replacement
Answers
In the Haber Process, Ammonia is created. The balanced reaction is as follows: 3H2 + N2 ⇒ 2NH3. The reaction is decomposition reaction. Therefore, option A is correct.
What is decomposition reaction ?A decomposition reaction occurs when a compound is broken down into two or more simpler substances. A decomposition reaction has the general form: ABA+B. The majority of decomposition reactions require an energy input in the form of heat, light, or electricity.
Carbonates are broken down into carbon dioxide and an oxide. Chlorate is broken down into oxygen gas and chloride. Water and an oxide are formed when hydroxides decompose. Water and a molecular oxide are formed when oxygen-containing acids decompose.
Thus, option A is correct.
To learn more about the decomposition reaction, follow the link;
https://brainly.com/question/16987748
#SPJ1
what feature related to the composition of a comopund can be determined solely by percent composisi
Answers
The % composition of a substance can be used to calculate empirical formulas. The molar mass of the chemical must also be known in order to determine its molecular formula.
What is empirical formula explain?
A compound's various atoms are arranged in an empirical formula in the simplest whole-number ratio.
The exact amount of various atom types that make up a compound's molecule are displayed in the molecular formula. The empirical formula for acetylene is CH. Example: C2H2 is the empirical formula for acetylene.
An illustration of an empirical formula?
Its chemical name is C6H12O6, and it is a simple sugar. Every mole of carbon and oxygen is accompanied by two moles of hydrogen. Glucose's empirical formula is CH2O.
Learn more about empirical formula
brainly.com/question/14044066
#SPJ4
If a wave of red light has a wavelength of 6.7 x 10-7 m, will the frequency of the red wave be high or low?
Answers
Answer:
Its high
Explanation:
Becuase if u times it what do u get
Which shows the correct order of stages of technological design?
Answers
Answer:
The four stages of technological design include identifying a need, designing and implementing a solution, and evaluating the solution.
I don't know what the options are, cause you didn't show them but, hope this helped.
Answer:
The correct answer is:C. Identify a problem, design a solution, implement the solution, evaluate the solution
Explanation:
when 13.8 ml of 0.870 m lead(ii) nitrate reacts with 90.0 ml of 0.777 m sodium chloride, 0.279 kj of heat is released at constant pressure. what is δh° for this reaction?
Answers
At constant pressure, 0.279 kg of of heat are produced if 13.8 ml of got its start m lead(ii) nitrate interacts and 90.0 ml of stars m chloride, releasing 23.3 kJ.
What is an illustration of pressure?An easy way to demonstrate pressure is to put a knife against some fruit. If you place the flat part of the blade against the fruit, the skin won't be cut. A large area is covered either by energy (low pressure).
Describe pressure.The physiological force applied to an object is referred to as pressure. Per unit area, a perpendicular force is delivered to a surface of the objects. F/A seems to be the primary formula for pressure (Force per unit area). Pascals are a pressure unit (Pa).
To know more about Pressure visit:
https://brainly.com/question/22613963
#SPJ4
The complete question is-
When 13.8 mL of 0.870 M lead(II) nitrate reacts with 90.0 mL of 0.777 M sodium chloride,0.279 kJ of heat is released at constant pressure.
What is ΔH° for this reaction?
Pb(NO3)2(aq) + 2NaCl(aq) → PbCl2(s) + 2NaNO3(aq)
(a). 23.3kJ
(b). 8kJ
(c). 1.84kJ
(d). 3.41kJ
(e). 4kJ
sterile isopropyl alcohol (ipa) bottles did not arrive in the supply order. what action should compounding personnel take?
Answers
Compounding personnel should contact the supplier to follow up on the delivery of the sterile isopropyl alcohol (IPA) bottles that did not arrive in the supply order. The personnel should request an estimated time of arrival and make sure to provide the exact details of the order.
If the sterile isopropyl alcohol (IPA) bottles did not arrive in the supply order, the compounding personnel should take the following steps:
It is important for the compounding personnel to take swift action in this situation to ensure that the necessary supplies are obtained and that compounding activities can continue without disruption.
know more about isopropyl alcohol here
https://brainly.com/question/14896958#
#SPJ11
If an object is on top of a hill or in a position raised above the ground, it must have __________.
Answers
Answer:
The force applied to the object is an external force, from outside the system. When it does positive work it increases the gravitational potential energy of the system. Because gravitational potential energy depends on relative position, we need a reference level at which to set the potential energy equal to 0.
If an object is on top of a hill or in a position raised above the ground, it must have gravitational potential energy.
What is Potential Energy ?
In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors
If an object is lifted, work is done against the force of gravity.
When work is done energy is transferred to the object and it gains gravitational potential energy.
If the object falls from that height, the same amount of work would have to be done by the force of gravity to bring it back to the Earth’s surface.
Due to the Principle of Conservation of Energy we can say that:
Gravitational potential energy at the top = kinetic energy at the bottom
To know more about Potential Energy
https://brainly.com/question/24284560
#SPJ2
Neon is located in the last group of the periodic table. How many valence electrons does the element neon have? (5 points)
1
2
4
8
Answers
Answer:
it's 8
Explanation:
the last group of the periodic table is group 8. so all elements in a group should have the same number of valency electrons
2. 3.80 km to meters
T
Answers
2.380km= 2e+3m.
A kilometer is a unit of measurement, as is the mille. It additionally translated as 10,000-steps meters, creating a strolling aim that, thru the decades, by some means has become embedded in our global awareness and fitness trackers. however contemporary exceptional science suggests we do now not need to take 10,000 steps an afternoon, which is ready five miles, for the sake of our health or toughness.
The CDC recommends that on foot 10,000 steps a day is sufficient to stay in a suitable form, but the general public needs to hit 20,000 steps as a part of their fitness desires. if you need to try the 20000 steps a day weight reduction, you can expect notable results: decreased belly fat, progressed sleep, boosted immune response, and so on. As excellent as it sounds, taking walks 20000 steps a day of weight loss has its cons. They include emotions of tiredness and starvation, plus it steals a big chew of your day.
Learn more about Meter here:-https://brainly.com/question/790324
#SPJ9
The half-life of a sample of asatine As-210 is about 8 hours. How
many half-lives must pass for only 25 grams of a 200-gram
sample to remain?
Answers
Answer:
3
Explanation: