Answers
Catalyst has no effect on the amount of product formed at the end of the reaction. Option D is correct
Explanations:What is a catalyst?A catalyst is. substance that speed up the rate of chemical reaction thereby causing the product to be formed within a short period of time. Some of the importance of catalyst include:
• They make reaction occur very fast
,• Lower the activation energy of a reaction
,• Create alternative pathway for reaction to proceed
Catalyst does not necessarily increase the amount of product formed but make it form within a short period.
Also they only provide alternative pathway but not having lower activation energy (They only lower the activation energy)
Based on the explanation, we can conclude that catalyst has no effect on the amount of product formed at the end of the reaction.
Related Questions
please help
A 43.40 mg sample of an alcohol
contains 15.10 mg O, 22.6 mg C, and
the rest is hydrogen.
What is its
percent composition of carbon?
[ ? ]% C
Round your answer to the hundredths place
Answers
The percentage composition of the carbon in the sample of alcohol is 52.1 %.
What is the percentage composition?The percentage of an element in a given compound can be determined from the number of parts by mass of that element present in 100 parts by mass of the given compound.
First, calculate the total mass of the compound then, the total mass of the element present in the compound is divided by the total mass of the compound multiplied by 100.
Given, the mass of oxygen, and carbon, is 15.10 mg, and 22.6 mg respectively.
The total mass of the sample = 43.40 mg
The mass of the hydrogen = 43.40 - (15.10 + 22.6) = 5.7 mg
The percentage composition of the carbon = (22.6/43.40) × 100 = 52%
Therefore, the percentage composition of the carbon is equal to 52.1 %.
Learn more about the percentage composition, here:
brainly.com/question/11952337
#SPJ1
10 points
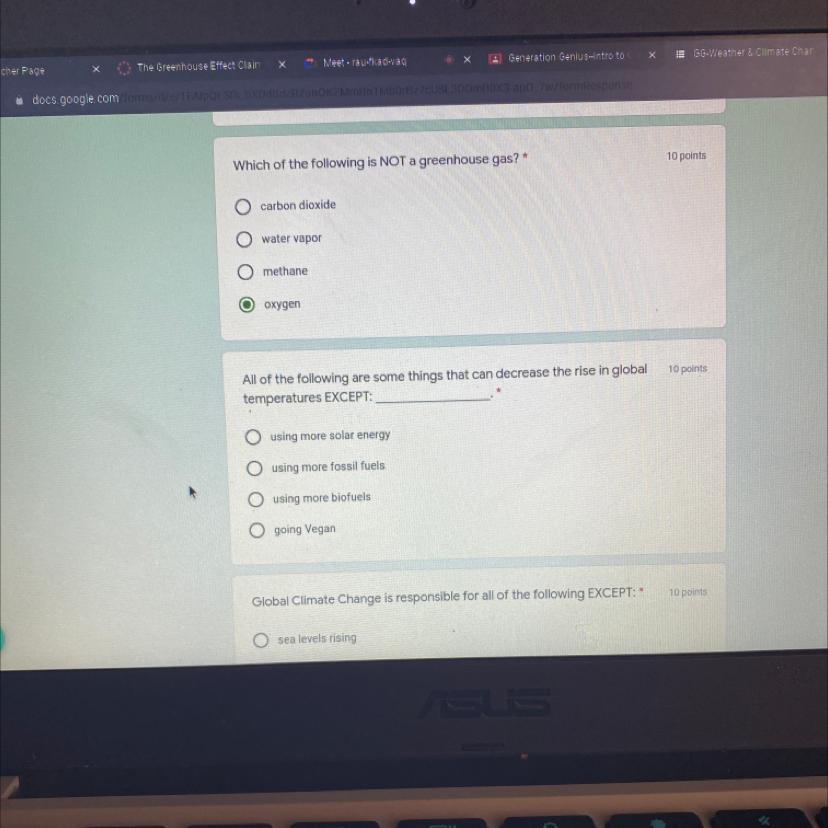
All of the following are some things that can decrease the rise in global
temperatures EXCEPT:
using more solar energy
using more fossil fuels
using more biofuels
going Vegan
Answers
Answer: Using more fossil fuels
Explanation: Burning fossil fuels releases Green House gasses into the atmosphere, such as carbon dioxide. The carbon dioxide in the atmosphere traps in heat, which causes global temperatures to increase.
Pewter is a solidified solution of tin and lead or tin and zinc. In both cases, tin is the main component. Which metal would you classify as the solute in each type of pewter?
Answers
(a) A compound X contains carbon, 66.7% and hydrogen 11.1% the rest being oxygen. Determine the empirical formula of X. (b) The vapor density of X is 36; determine the molecular formula of X.
Answers
Answer:
The molecular formula of X = C4 h8 O
7. What is the volume of the
composite
solid?
4 in.
3 in.
3 in.

Answers
Answer:
The volume of Component 1 is 36 cubic inches.
Explanation:
To calculate the volume of a composite solid, we need to determine the individual volumes of the different components and then add them together.
In this case, the composite solid consists of multiple components with the following dimensions:
Component 1:
Length: 4 inches
Width: 3 inches
Height: 3 inches
To find the volume of Component 1, we multiply the length, width, and height together:
Volume of Component 1 = Length x Width x Height = 4 in x 3 in x 3 in = 36 cubic inches
Therefore, the volume of Component 1 is 36 cubic inches.
Please provide the dimensions of the remaining components of the composite solid, and I will calculate the total volume by summing up the individual volumes.
7.Which one of the following statements is not true?
1 point
O The molecules in a solid vibrate about a fixed position
O The molecules in a liquid are arranged in a regular pattern
The molecules in a gas exert negligibly small forces on each other, except during
collisions
The molecules of a gas occupy all the space available
Answers
Answer:
B. the molecules in liquid are loosely packed and scattered thus, they cannot be arranged
A 0.5998g sample of a new compound has been analyzed and found to contain the following masses of elements: carbon = 0.1.565g ; hydrogen = 0.02627g ; oxygen = 0.4170g. Calculate the empirical formula of the compound.
Answers
The empirical formula of a novel compound is CH2O if an analysis of a 0.5998g sample reveals that it contains the following masses of elements: carbon (0.1.565g), hydrogen (0.02627g), and oxygen (0.4170g).
What's in a 23.0 g sample of a substance?You need to be aware of a compound's molar mass in order to ascertain its molecular formula. You can then decide which multiple of the empirical formula corresponds to the right molecular formula. 12.0g of carbon, 3.0g of hydrogen, and 8.0g of oxygen make up a compound in a 23.0g sample.
Which chemical has an empirical formula that is 92.3% carbon and 7.7% hydrogen?In a hydrocarbon, carbon makes up 92.3% of the mass and hydrogen makes up 7.7%.
To know more about empirical formula visit:-
https://brainly.com/question/14044066
#SPJ1
Which element in Period 3 of the Periodic Table has the largest atomic radius?
A . CI
B.P
C.Na
D. AI
Answers
Answer:
NA
Explanation:
Determine the type of alcohol corresponding to each given description or name. 1-pentanol 3-ethyl-3-pentanol 2-hexanol An alcohol with two other carbons attached to the carbon with the hydroxyl group_____.An alcohol with one other carbon attached to the carbon with the hydroxyl group____.An alcohol with three other carbons attached to the carbon with the hydroxyl group____.
Answers
Answer:
1). 1-pentanol - Primary
2). 3-ethyl-3-pentanol - Tertiary
3). 2-hexanol - Secondary
4). Alcohol with two other carbons attached to the carbon with the hydroxyl group - Secondary
5). Alcohol with one other carbon attached to the carbon with the hydroxyl group - Primary
6). Alcohol with three other carbons attached to the carbon with the hydroxyl group - Tertiary
Explanation:
The distinct types of alcohol have been matched with the categories above as per their descriptions provided. In chemistry, alcohols have been categorized into three different categories namely primary, secondary, and tertiary.
In the primary type, those alcohols are involved in which there is an association of hydroxyl group to a primary atom of carbon along with a minimum of two atoms of hydrogen. Example; ethanol.
In the secondary type, the alcohols have an association of carbon atoms to hydroxyl with a single atom of hydrogen and has a formula of '-CHROH.' Example: 2 - propanol.
In the tertiary alcohols, here the association is between the hydroxyl group with the carbon atom that is saturated and possessing 3 atoms of carbon associated with it. It has a formula of '-CR2OH.' Example: 3-ethyl-3-pentanol, -tert -butyl alcohol, etc.
Line p has an equation of y= – 8x+6. Line q, which is perpendicular to line p, includes the point (2, – 2). What is the equation of line q?
Answers
The equation of line q is determined as y = ¹/₈x - ⁹/₄.
What is slope?The slope of a line is the change in y axis to change in x axis.
Slope of line Py = – 8x + 6
from general line equation, y = mx + c
where;
m is slopem = - 8
Slope of line qm₂ = -1/m
m₂ = -1/-8
m₂ = 1/8
Equation of line q(y - y₁) / (x - x₁) = m₂
(y + 2)/(x - 2) = 1/8
y + 2 = ¹/₈(x - 2)
y + 2 = ¹/₈x - ¹/₄
y = ¹/₈x - ⁹/₄
Thus, the equation of line q is determined as y = ¹/₈x - ⁹/₄.
Learn more about equation of line here: https://brainly.com/question/13763238
#SPJ1
To what temperature would 12.3g of He have to be cooled to fit a 34.0 l tank at 1.17 atm?
Answers
The ideal gas law may be used to calculate the temperature at which 12.3 g of He would need to be cooled to fit a 34.0 l tank at 1.17 atm. According to this rule, the ideal gas constant multiplied by the number of moles in the gas equals the product of an ideal gas's pressure, volume, and temperature.
In order to solve for temperature, we may thus rearrange the equation. PV = nRT, where P is for pressure, V is for volume, n is for moles, R is for the ideal gas constant, and T is for temperature, is the equation. By multiplying both sides of the equation by the inverse of P and dividing both sides by nR, we may find the value of T.This results in the formula T = PV/nR.
The values for P, V, and nR can be substituted for the predetermined conditions. The volume is 34.0 l and the pressure is 1.17 atm. By dividing the mass, 12.3 g, by He's molar mass, 4.00 g/mol, the amount of moles of He may be computed. 0.0821 L*atm/K*mol is the ideal gas constant. Plugging these values into the equation gives us a temperature of about -267.7 K.
Learn more about temperature at:
https://brainly.com/question/11464844
#SPJ1
how many elements are in 3caso4
Answers
Answer:
there are only three elements in 3CaSO4: calcium, sulfur and oxygen
what is the molality of a solution that contains 63.0 g HNO3 (Nitic acid) in 0.500 kg H2O?
Answers
The first step to answer this question is to use the molar mass of HNO3 to convert the given grams of this substance to moles (MM=63.0g/mol):
\(63.0g\cdot\frac{mol}{63.0g}=1mol\)Now, divide the moles of solute by the kg of solvent to find the molality of the solution:
\(m=\frac{1mol}{0.500kg}=2m\)The molality of the solution is 2m.
When a chemical reaction occurs blank happens
Answers
Answer:
In a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to make the products.
Explanation:
What amount of heat, in kJ, is required to vaporize 181.20 g of ethanol (C₂H₅OH)? (∆Hvap = 43.3 kJ/mol)
Answers
The amount of heat required to vaporize 181.20 g of ethanol would be 170.1 kJ.
Heat of vaporizationUsing the formula:
Q = n ∆Hvap
where:
Q is the amount of heat required to vaporizen is the number of moles of the substance∆Hvap is the molar heat of vaporization.Moles of 181.20 g of ethanol = 181.20 g / 46.07 g/mol = 3.933 mol
Substituting the values:
Q = 3.933 mol x 43.3 kJ/mol = 170.1 kJ
In other words, the amount of heat required to vaporize 181.20 g of ethanol is 170.1 kJ.
More on heat of vaporization can be found here: https://brainly.com/question/30603212
#SPJ1
Which of the following is a balanced equation for the reaction of aluminum, Al, and hydrochloric acid, HCl, to form aluminum chloride, AlCl3, and hydrogen, H2?
a. Al + 3 HCl → AlCl₃ + H₂
b. Al + 6 HCl → AlCl³ + 3 H₂
c. 2 Al + 6 HCl → 2 AlCl₃ + 3 H₂
d. Al + 6 HCl → 2 AlCl₃ →3 H₂
Answers
The balanced equation for the reaction of aluminum, Al, and hydrochloric acid, HCl, to form aluminum chloride, AlCl3, and hydrogen, H2 is Al + 3 HCl → AlCl₃ + H₂. hence, the correct option is (a).
This equation shows that 1 mole of aluminum reacts with 3 moles of hydrochloric acid to produce 1 mole of aluminum chloride and 1 mole of hydrogen gas. The coefficients on both sides of the equation are balanced, indicating that the law of conservation of mass is satisfied. In order to satisfy the law of conservation of mass, a chemical equation must be balanced, which means that the number of atoms of each element must be the same on both sides of the equation. The coefficients in front of each compound or element indicate how many moles of that substance are involved in the reaction. By adjusting the coefficients in the equation, we can balance the number of atoms of each element and ensure that the total mass of the reactants equals the total mass of the products.
To know more about reaction please refer: https://brainly.com/question/4482869
#SPJ4
How many grams of K2Cr2O7 can be dissolved at 279 grams of water at 90°C?
Answers
Answer:
How many grams of K2Cr2O7, are soluble in 100 g of water at 95 ºC? Solubility Curve DRAFT. 10th - 12th grade. 3326 times. Chemistry. 63% average accuracy. 3 years ago ... When 20 grams of potassium chlorate, KClO 3, is dissolved in 100 grams of water at 80 ºC, the solution can be correctly described as: answer choices . supersaturated. saturated.
Explanation:
45. Assume that 0.504 g of H₂ gas at STP reacts with
excess CuO according to the following equation:
Cuo(s) + H₂(g) → Cu(s) + H₂O(g)
Make sure the equation is balanced before beginning
your calculations.
a. How many liters of H₂ react?
b. How many moles of Cu are produced?
C. How many grams of Cu are produced?
Answers
CuO(s) + H2(g) → Cu(s) + H2O(g)
a. To find the volume of H2 gas at STP, we can use the ideal gas law:
PV = nRT
At STP, the pressure (P) is 1 atm and the temperature (T) is 273 K. The volume (V) of 1 mole of gas at STP is 22.4 L. So, the number of moles (n) of H2 gas is:
n = PV/RT = (1 atm) x V/(0.0821 L·atm/mol·K x 273 K) = V/22.4
We know that 0.504 g of H2 gas reacts. The molar mass of H2 is 2 g/mol. So, the number of moles of H2 gas is:
n = m/M = 0.504 g/2 g/mol = 0.252 mol
Now we can find the volume of H2 gas:
V = n x 22.4 L/mol = 0.252 mol x 22.4 L/mol = 5.65 L
Therefore, 5.65 liters of H2 gas react.
b. From the balanced equation, we can see that the mole ratio of CuO to Cu is 1:1. So, the number of moles of Cu produced is also 0.252 mol.
c. To find the mass of Cu produced, we can use the molar mass of Cu, which is 63.5 g/mol:
mass of Cu = number of moles of Cu x molar mass of Cu
= 0.252 mol x 63.5 g/mol
= 16.02 g
Therefore, 16.02 grams of Cu are produced.
Which of the following equations correctly model a nuclear fusion reaction? Justify your reasoning

Answers
Answer:
Explanation:
c) fusion combines into one product plus energy
_______ is the measure of how much water vapor is in the air
Answers
Answer:
Humidity is the measure of how much water vapor is in the air.
calculate the concentration of HCL
Answers
the concentration of HCI
solution:\(mass \: formula \: of \: hci = 1 + 35.5\)
\( = 36.5\)
\(mass = relative \: formula \: mass \times amount\)
\( = 36.5 \times 0.125\)
\( = 4.56g | {dm}^{3} \)
hence, the concentration of HCI is 4.56g/dm3.
PLSS HELPP ME i dont knowww

Answers
Answer:
non polar. polar ionic substance
If 62.1 grams of magnesium react with 100.0 grams of magnesium, how many grams of product are produced?
Which is the Limiting reactant?
Which is the Excess reactant?
How many grams of excess reactant remain?
pls help me with homework
Answers
The limiting and excess reactant can be obtained as follow:
2Mg + O₂ -> 2MgO
Molar mass of Mg = 24 g/molMass of Mg from the balanced equation = 2 × 24 = 48 g Molar mass of O₂ = 32 g/molMass of O₂ from the balanced equation = 1 × 32 = 32 gFrom the balanced equation above,
48 g of Mg reacted with 32 g of O₂
Therefore,
62.1 g of Mg will react with = (62.1 × 32) / 48 = 41.4 g of O₂
We can see from the above that only 41.4 g of O₂ is required to react completely with 62.1 g of Mg.
Thus, the limiting reactant is magnesium, Mg and the excess reactant is oxygen, O₂
How do i determine the mass of the excess reactant remaining?The mass of the excess reactant remaining can be obtained as shown below:
Mass of excess reactant, O₂ given = 100 gMass of excess reactant, O₂ that reacted = 41.4 gMass of excess reactant, O₂ remaining =?Mass of excess reactant remaining = Mass given - mass reacted
Mass of excess reactant remaining = 100 - 41.4
Mass of excess reactant remaining = 58.6 g
How do i determine the mass of product produced?The mass of H₂SO₄ produced can be obtained as illustrated below:
Mg + O₂ -> 2MgO
Molar mass of Mg = 24 g/molMass of Mg from the balanced equation = 2 × 24 = 48 g Molar mass of MgO = 40 g/molMass of MgO from the balanced equation = 2 × 40 = 80 gFrom the balanced equation above,
48 g of Mg reacted to produce 80 g of MgO
Therefore,
62.1 g of Mg will react to produce = (62.1 × 80) / 48 = 103.5 g of MgO
Thus, the mass of product, MgO produced is 103.5 g
Learn more about mass produced:
https://brainly.com/question/9526265
#SPJ1
Complete question:
If 62.1 grams of magnesium react with 100.0 grams of oxygen, how many grams of product are produced?
Which is the Limiting reactant?
Which is the Excess reactant?
How many grams of excess reactant remain?
Oxygen and hydrogen are compressed into two cubical boxes of the same
size at a temperature of 28 K. What do these gases have in common
according to the kinetic theory?
Answers
Explanation:
Following are the kinetic theory of gases postulates:
1) Space-volume to molecules ratio is negligible.
2)There is no force of attraction between the molecules at normal temperature and pressure. The force of attraction between the molecules build when the temperature decreases and the pressure increases.
3) There is large space between the molecules resulting in continuous motion.
4) The free movement of molecules results in collision which is perfectly elastic.
5) The molecules have kinetic energy due to random movement. But the average kinetic energy of these molecules differs with temperature.
6) Molecules exert pressure on the walls of the container.
What changes occur in the sky over the period of 24 hours? Why do these changes occur?
Answers
Answer:
Orientation
Explanation:
When setting a budget, you should consider...
creative ways to spend your money.
mostly your goals.
financial goals, current expenses, and income.
mostly income.
Answers
Financial goals, current expenses, and income are the creative ways to spend your money.
What is budget?A budget represents a calculating plan, typically financial but not always, for a specific time frame, typically one year or one month. Predicted sales and revenue amounts, resource quantities (such as time, costs, and expenses), environmental impacts (such as greenhouse gas emissions), other impacts, assets, liabilities, and cash flows are all possible inclusions in a budget.
Budgets are a measurable way for businesses, governments, families, and numerous other organisations to articulate their strategic plans of action. In a budget, intended expenses are expressed together with suggestions for how to fund them. A budget can show a surplus of resources for later use or a deficit if expenses are greater than income as well as other resources. Financial goals, current expenses, and income are the creative ways to spend your money.
Therefore, financial goals, current expenses, and income are the creative ways to spend your money.
To know more about budget, here:
https://brainly.com/question/17198039
#SPJ2
A 4.50 L sample of neon at 11.08 atm is added to a 12.0 L cylinder that contains argon. If the pressure in the cylinder is 7.15 atm after the neon is added, what was the original pressure (in atm) of argon in the cylinder?
Answers
The original pressure of argon in the cylinder is 5.68 atm.
What is the original pressure of the argon?
The original pressure of argon in the cylinder is calculated as follows.
Apply the concept of molar ratio or molar proportion to determine the original pressure of argon in the cylinder.
Pr = [(Pn x Vn) + (Pa x Va) ] / (Vn + Va)
where;
Pr is the resultant pressure of the mixture of gases = 7.15 atmPn is the original pressure of neon = 11.08 atmPa is the original pressure of argonVn is the volume of neon = 4.5 LVa is the volume of argon = 12 L7.15 = [(11.08 x 4.5) + (12 x Pa)] / (4.5 + 12)
7.15(4.5 + 12) = 49.86 + 12Pa
117.98 = 49.86 + 12Pa
68.12 = 12Pa
Pa = 68.12/12
Pa = 5.68 atm
Thus, the original pressure of argon in the cylinder is determined by applying concept of molar ratio.
Learn more about original pressure of gas here: https://brainly.com/question/25736513
#SPJ1
what is the pH of a solution that contains 0.0425 moles of HCl in 6.50 litres of water?
Answers
Answer:
To find the pH of a solution that contains a strong acid like HCl, you can use the equation:
pH = -log[H+]
Where [H+] is the concentration of hydrogen ions in moles per liter. In this case, you know the concentration of HCl and the volume of water, so you can calculate the concentration of hydrogen ions by using the equation:
[H+] = (0.0425 moles HCl) / (6.50 liters water) = 0.0653846 moles/liter
Finally, you can calculate the pH:
pH = -log[0.0653846 moles/liter] = 1.18
So the pH of the solution is 1.18.
Explanation:
Do you want to know shot trick also?
Many ravines can be found in forested areas of the Cross Timbers ecoregion of Texas. What natural process most likely formed
these ravines?
OA. erosion by water during the rainy season
OB. erosion by wind during the dry season
OC. deposition by water during the rainy season
OD. deposition by wind during the dry season
Answers
Erosion by water during the rainy season can be a significant natural phenomenon. When it rains, water flows over the land, picking up soil particles and carrying them along with it.
What is water ?Water is a clear, colorless, odorless, and tasteless liquid that is essential for life. It is a chemical compound made up of two hydrogen atoms and one oxygen atom, with the chemical formula H2O. Water is the most abundant substance on Earth, covering approximately 71% of its surface.
Water is a unique substance because of its molecular structure, which allows it to exist in all three physical states - solid, liquid, and gas - at temperatures and pressures commonly found on Earth. It has a high heat capacity, meaning it can absorb and release large amounts of heat without changing temperature significantly. This property is important for regulating the Earth's climate and for maintaining the temperature of living organisms.
Water is essential for many biological processes, such as hydration, digestion, and metabolism. It also plays a crucial role in the Earth's water cycle, where it evaporates from bodies of water and plants, condenses into clouds, and falls back to the surface as precipitation.
To know more about Water visit :
https://brainly.com/question/28465561
#SPJ1
What sample at STP has the same number of molecules as 5 L of NO2
Answers
Answer:
5l NO
2
at STP
No. of molecules=
22.4
5
mol=
22.4
5
×N
A
molecules
A) 5ℊ of H
2
(g)
No. of moles=
2
5
mol=
2
5
×N
A
molecules
B) 5l of CH
4
(g)
No. of moles of CH
4
=
22.4
5
mol=
22.4
5
N
A
molecules
C) 5 mol of O
2
=5N
A
O
2
molecules
D) 5×10
23
molecules of CO
2
(g)
Molecules of 5l NO
2
(g) at STP=5l of CH
4
(g) molecules at STP
Therefore, option B is correct.
Was this answer helpful?
