what is the product(s) in the reaction below
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Answers
Answer:
Aqueous sodium hydroxide and hydrogen gas
Related Questions
I need help with this. Thank youuuuuuu

Answers
The number of atoms in 3 moles K of the particle is 1.806 x 10²⁴ atoms.
What is the number of atoms in the given moles?
The value of one mole of an atom is equal to exactly 12 grams of pure carbon-12.
12.00 g (C-12 ) = 1 mol C-12 atoms = 6.02 × 10²³ atoms .
The number of particles in 1 mole is called Avogadro's Number (6.0221421 x 10²³).
The number of atoms in 3 moles K of the particle is calculated as follows;
= 3 moles x 6.02 × 10²³ atoms / mol
= 1.806 x 10²⁴ atoms
Learn more about number of atoms here: https://brainly.com/question/6258301
#SPJ1
Select the correct answer. a student puts a glass of water in the freezer. later, he notices ice forming on the surface of the water. which property of water best explains why ice forms on its surface?
Answers
Please help and right down each step!!

Answers
The mass of \(Ag_2CrO_4\) that would be formed will be 2.69 grams.
Stoichiometric problemMole of 2.5 grams silver nitrate = 2.5/143.32 = 0.017 mol
Mole of 18.0 mL, 0.450 M sodium chromate = 18/1000 x 0.450 = 0.0081
From the balanced equation of the reaction, the mole ratio of silver nitrate to sodium chromate is 2:1. In other words, the sodium chromate is a bit limiting.
The mole ratio of sodium chromate and \(Ag_2CrO_4\) is 1:1. Hence, the equivalent mole of \(Ag_2CrO_4\) formed would also be 0.0081 mol.
Mass of 0.0081 mol \(Ag_2CrO_4\) = 0.0081 x 331.73
= 2.69 grams
In other words, the mass of \(Ag_2CrO_4\) formed from the reaction would be 2.68 grams.
More on stoichiometric problems can be found here: https://brainly.com/question/29856106
#SPJ1
what does command mean
Answers
Answer:
TechnologyCommand Data Solutions specializes in empowering your data and content with a high degree of quality and scope. Intelligent data fine-tunes your advertising, and enables your core sales team to work within relevant market zones. This saves a lot of valuable time and increases ROI.
Explanation:
Answer:
Technology Command Data Solutions specializes in empowering your data and content with a high degree of quality and scope. Intelligent data fine-tunes your advertising, and enables your core sales team to work within relevant market zones. This saves a lot of valuable time and increases ROI. We do this with a multi-faceted approach using cutting edge technologies. We also market your content through various channels such as telemarketing and e-mail campaigns, as well as syndication. We further engage your customers through blogs, webinars and webcasts. We closely study your customers’ requirements and arrange meetings when the time is right. This ground work frees up your sales force to focus on its strength: hard core sales. We cleanse your existing database and remove irrelevant data. We enrich it with new, more relevant contact information. We monitor your existing and potential customer profiles and track their technology needs. The moment we sense purchase behavior, you get an instant update.
Sodium chloride is a soluble salt that can be made by mixing sodium hydroxide with an acid. Sodium metal cannot be used because it is too... what?
Answers
Answer:
Reactive.
Explanation:
am sure bro..…….
Because it is too reactive, sodium metal cannot be used to make sodium chloride. Since sodium is a highly reactive alkali metal, it easily interacts with other substances to form new ones.
What is the intraction of the sodium ?Sodium metal interacts fast with water to produce sodium hydroxide and hydrogen gas. The metal may catch fire and burn as a result of this reaction since it is so exothermic, or heat-producing.
Sodium metal interacts fast with acids like hydrochloric acid to produce sodium chloride and hydrogen gas. However, handling this reaction can be risky due to its high exothermic nature.
There is a significant safety risk since the heat from the reaction might cause the acid to boil and spray.Boil and spatter poses a major risk to people's safety. In comparison, using sodium hydroxide to create sodium chloride is significantly safer.
When sodium hydroxide and an acid are combined, the reaction is significantly slower and less exothermic. This response is considerably simpler to manage and does not provide a significant risk to safety. Sodium hydroxide is also considerably simpler to get than sodium metal. As a result, it is the technique of choice for producing sodium chloride.
Learn more about exothermic reactions at:
https://brainly.com/question/28546817
#SPJ2
Chemical Formula:
4Ca(OH)2
In the chemical formula above, how many different types of elements are present in a molecule of this formula?
Answers
Answer:
They are just two....Ca and OH
olive oil (density 0.918 g/cm3) separates from the water-vinegar in a salad dressing and is visible as a layer. will the water be the top or the bottom layer? why?
Answers
Olive oil separates from the water-vinegar in a salad dressing and is visible as a layer because the olive oil will be on the top because it has lesser density.
The density of a substance indicates how dense it is in a particular area. Mass per unit volume is the definition of a material's density. In essence, density is a measurement of how closely stuff is packed. It is a special physical characteristic of a certain thing.
On top of the liquid with higher density, the liquid with lower density will float. Compared to water, oil has a lower density. Hence, it floats on top of it. Oil is a non-polar liquid, but water is polar, hence there will be no attraction between their molecules.
Learn more about Density:
https://brainly.com/question/13632227
#SPJ4
A sample of gas has a mass of 0.545 g. Its volume is 119 mL at a temperature of 85 degrees Celsius and a pressure of 720 mmHg. Find the molar mass of the gas.
Absolute Temperature:
When solving problems with gases, it is important to convert temperature to the absolute kelvin scale. The term "absolute" in the context of measurement scales means that zero is the lowest possible number in the scale. Celsius is not an absolute scale as its measurements are relative to the melting/freezing point of water, making negative values for temperatures possible on the scale.
Answers
the molar mass of the gas comes out to be 137.28 g/mol.
We can apply the ideal gas law equation to determine the gas' molar mass:
PV = nRT
where P is for pressure.
V = volume and n = moles.
Ideal gas constant: R
Temperature is T.
Let's first translate the provided values into SI units:
Pressure (P) is defined as 720 mmHg, 720 torr, or 720/760 atm.
Volume (V) = 0.119 L/119 mL
85 degrees Celsius is equal to 85 + 273.15, or 358.15 Kelvin.
The ideal gas law equation is then rearranged to account for the number of moles (n):
n = PV / RT
n = (720/760) atm * 0.119 L / (0.0821 Latm/molK) * 358.15 K can be substituted for the original values.
Condensing: n 0.00512 mol
Now, we may use the following formula to determine the gas's molar mass (M):
M is equal to mass / moles.
Changing the numbers to: M = 0.545 g / 0.00512 mol
Putting it simply: M = 106.64 g/mol
As a result, the gas's molar mass is roughly 106.64 g/mol.
to know more about the ideal gas law refer to the link below
https://brainly.com/question/4147359
#SPJ4
Determine which elements are A and B for the molecule butane.
A Choose...
B Choose...


Answers
Answer:
The answer is
A- H
B- C
Explanation:
Answer:
The answer is
A- H
B- C
Explanation:
Determine the mass in grams of 0.69 mol of gold (Au)
Answers
Answer:
We assume you are converting between moles Gold and gram. You can view more details on each measurement unit: molecular weight of Gold orgrams The molecular formula for Gold is Au. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Gold, or 196.96655grams.
what is the limiting reagent in the chemical reaction that was used to determine the molar volume of h2 gas?
Answers
Magnesium is the limiting reagent in the chemical reaction that was used to determine the molar volume of H₂ gas.
The limiting reagent is the reactant that is completely used up in a reaction, and thus determines when the reaction stops.
Metals that are above hydrogen in the activity series will displace from an acid and produce hydrogen gas. Magnesium is an example of a metal that is more active than hydrogen in the activity series.
The reaction is,
Mg + HCl ---> MgCl₂ + H₂
The experiment is designed such that the magnesium metal is the limiting reagent.
To know more about the limiting reagent, here
brainly.com/question/26905271
#SPJ4
Which missing item would complete this beta decay reaction?

Answers
Answer:
The answer is option A.Hope this helps you
Potassium atoms contribute one eletron to metallic bonding,but calcium atoms contribute two eletrons .Explain which metal is likely to be harder
Answers
Answer:
potassium is likely to be harder
Explanation:
because whenan atom loss electron they can form particle ion acquare stable a arrangement that loos electron can transfer to another which that also to acquaire stable
i need help pls!!!!!!!!!!!
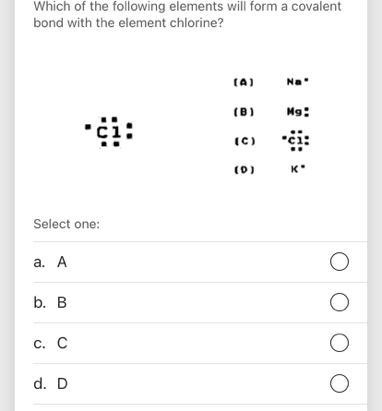
Answers
Which element will form covalent bonds with chlorine?
A. Carbon. YES. C is a nonmetal. B. Magnesium. NO. Mg is a metal. C. Potassium. NO. K is a metal. D. Aluminum. NO. Al is a metal.
Chlorine will form covalent bonds with A. Carbon .

Sugar is a compound made of the elements hydrogen, carbon, and oxygen. Which element is left on the spoon after the sugar is heated?
Answers
Answer:
carbon is the element left on the spoon after the sugar is heated.
what is the atomic number of silver
Answers
Answer:
47
Explanation:
Silver (Ag), chemical element, a white lustrous metal valued for its decorative beauty and electrical conductivity. Silver is located in Group 11 (Ib) and Period 5 of the periodic table
The atomic number of silver is 47.
That also means that silver has 47 electrons and 47 protons.
The atomic mass of silver is 108u.
107 - 47 = 60
Silver has 60 neutrons.
Which nitrogen base sequence is the partner of T-C-A-G-C-A?
• A-C-G-A-C-T
• C-A-G-A-T-G
• A-G-T-C-G-T
• T-C-A-G-C-A
Help pls
Answers
According to the complementary base pairing in DNA, the nitrogen base sequence which is the partner of T-C-A-G-C-A is A-G-T-C-G-T.
What is DNA?DNA is a hereditary material which is present in human beings as well as all other living organisms. Every cell which is present in an organism's body has DNA which is the same. Most of the DNA is situated in the cell's nucleus and small amount of it can be found in the cell's mitochondria as well.
Information which is stored in DNA is stored as codes made up of four chemical bases namely, adenine, thymine , cytosine and guanine.Human DNA consists of 3 billion bases .The order of the bases determines information which is required for building and maintaining an organism.
DNA bases are capable of pairing up with each other. Adenine pairs with thymine and guanine pairs up with cytosine .Each base is also attached to a sugar molecule and a phosphate group. A base, phosphate sugar are together called as nucleotides.
Learn more about DNA,here:
https://brainly.com/question/14315652
#SPJ9
What is the correct formula for the ionic compound chromium (III) nitride?
Answers
hope that helps
hi help plz .........

Answers
Answer:The answer is C) The total mass of C and D only.
Conduction can occur within a single substance, but parts of the substance must have which property?
Answers
In order for conduction to occur within a single substance, parts of the substance must have high thermal conductivity.
Conduction is a process of heat transfer where heat is transferred from hotter particles to colder ones. Conduction can occur within a single substance, but parts of the substance must have high thermal conductivity. This means that the molecules in these parts of the substance are closely packed together and can easily transfer heat energy to one another. The rate of heat conduction within a substance depends on several factors, including the temperature difference between the hotter and colder parts of the substance, the thickness of the substance, and the thermal conductivity of the substance itself.
The higher the thermal conductivity of the substance, the faster heat will be transferred from one part of the substance to another. Therefore, in order for conduction to occur within a single substance, it is necessary that parts of the substance have high thermal conductivity. This ensures that heat can be transferred efficiently from one part of the substance to another, resulting in an equalization of temperature throughout the substance.
https://brainly.com/question/23897839
To know more about thermal conductivity visit:-
#SPJ11
The contents of a Helium ballon are in which phase? A. Solid B. Plasma C. Liquid G. Gas
Answers
A sample of gas has a volume of 215 cm3 at 23.5 °C and 3 atm. What will the volume of the gas be at STP
Answers
Answer:
165.3 cm^3
Explanation: hope this is correct!!
P1 * V1 / T1 = P2 * V2 / T2
P1 = 84.6 kPa
V1 = 215 cm³
T1 = 23.5°C = 23.5 + 273 K = 296.5 K
At STP:
P2 = 101.3 kPa
V2 = ?
T2 = 273 K
What is one property of salts?
OA. They have a low pH.
O B. They are neutral.
O C. They have a high pH.
OD. They are very reactive.
Answers
HELP.....................................................

Answers
Answer:
I believe that would be a liquid.
Explanation:
a reaction has a rate constant of 0.0117/s at 400.0 k and 0.689/s at 450.0 k. determine the activation barrier for the reaction in kj/mol. do not include units in your answer.
Answers
The activation barrier for the reaction in kJ/mol is ≈ 78.
The activation barrier for the reaction in kJ/mol can be calculated by using the Arrhenius equation.
The Arrhenius equation is represented by the following expression:
\(k = A^(^-^E^a^/^R^T^)\)
Where k = rate constant
A = frequency factor (pre-exponential factor)
Ea = activation energy
R = gas constant
T = temperature
In the equation, the exponential term represents the probability of reactant molecules possessing enough energy to react. The activation energy (Ea) is the minimum energy required to initiate the reaction. The frequency factor represents the probability of a successful collision between reactant molecules. It is assumed that the frequency factor is constant within a given temperature range. The rate constant is a measure of the reaction rate.The activation barrier for the reaction in kJ/mol is given by the following expression:
Ea = (R)(ln(k2/k1))/(1/T1 - 1/T2)
Where k1 and k2 are the rate constants at temperatures T1 and T2, respectively.
R is the gas constant.
Here, k1 = 0.0117/s, k2 = 0.689/s, T1 = 400.0 K, T2 = 450.0 K and R = 8.314 J/K mol
Converting the units of R to kJ/K mol,
R = 8.314/1000 = 0.008314 kJ/K mol
Therefore, the activation barrier for the reaction in kJ/mol is given by the expression:
Ea = (0.008314 kJ/K mol) × ln (0.689/0.0117) / ((1/400.0 K) - (1/450.0 K)) ≈ 78 kJ/mol
Learn more about rate constant: https://brainly.com/question/26724488
#SPJ11
A model depicting burning a mole of hydrogen and a mole octane
Answers
More energy is required to break the bonds in octane, and more energy is released when the products are formed.
Why does the combustion of octane produce more energy than the combustion of hydrogen?Burning one mole of hydrogen gas (H2) and one mole of octane (C8H18) yields different amounts of energy and products. The balanced chemical equations for the combustion of hydrogen and octane are:
Hydrogen: 2H2 + O2 -> 2H2O + energy (where energy is released in the form of heat)
Octane: C8H18 + 12.5O2 -> 8CO2 + 9H2O + energy (where energy is also released in the form of heat)
When one mole of hydrogen is burned, it releases 241.8 kJ of heat energy, whereas when one mole of octane is burned, it releases 5471 kJ of heat energy.
The combustion of octane produces more energy because it contains more carbon atoms than hydrogen atoms, and carbon-carbon bonds are stronger than carbon-hydrogen bonds.
As a result, more energy is required to break the bonds in octane, and more energy is released when the products are formed.
Learn more about Combustion.
brainly.com/question/15117038
#SPJ11
Please help with thesee!

Answers
Answer:
Compound X is named propene.
i. What happens when hydrogen bromide is added to the compound X?
When hydrogen bromide is added to an alkene, it will add across the double bond to form a new sigma bond. In the case of compound X, this would produce 2-bromopropane.
Reaction:
C3H6 + HBr → C3H7Br
ii. How can you prepare compound X from 1-chloropropane?
Compound X can be prepared from 1-chloropropane by reacting it with sodium hydroxide in water. The hydroxide ion will abstract a proton from the chlorine atom, leaving behind a negative charge on the carbon atom. This negative charge can then be attacked by a water molecule to form an alcohol.
Reaction:
CH3CH2CH2Cl + NaOH → CH3CH2CH2OH
iii. How can you prove chemically the compound X is unsaturated?
One way to prove that compound X is unsaturated is to react with bromine water. Bromine water is a solution of bromine in water. When bromine water is added to an unsaturated compound, the bromine will be added across the double bond to form a precipitate of bromine. This is a positive test for an unsaturated compound.
Reaction:
C3H6 + Br2 → C3H6Br2
iv. Convert methane into ethane.
Methane can be converted into ethane by reacting it with hydrogen gas in the presence of a catalyst, such as nickel. This reaction is called the Fischer-Tropsch process.
Reaction:
CH4 + H2 → C2H6
v. How can you prepare ethane from Kolbe's electrolysis?
Ethane can be prepared from Kolbe's electrolysis by passing an electric current through a solution of sodium acetate. The electric current will cause the acetate ions to decompose into carbon and hydrogen gas. The carbon and hydrogen gas will then react to form ethane.
Reaction:
2CH3COO- → C2H6 + 2CO2
I hope this helps! Let me know if you have any other questions.
Copper used in electric wires comes in two flavors (isotopes): Cu-63 and Cu-65. Cu-63 has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, Cu-65, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu.
Calculate the actual atomic mass of Cu-65.
Answers
Copper used in electric wires comes in two flavors (isotopes): Cu-63 and Cu-65. Cu-63 has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, Cu-65, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu is 64.93 amu.
Average atomic mass = sum of masses of isotopes multiply by % of abundance
Cu - 63, mass = 62.9298 amu, % abundance = 69.09 %
Cu - 65, mass m2 = ? % abundance = 30.91 %
average atomic mass = 63.546 amu
average atomic mass = ( 62.9298 × 0.6909 ) + ( m2 × 0.3091 )
63.546 = ( 62.9298 × 0.6909 ) + ( m2 × 0.3091 )
m2 = 64.93 amu
Thus, Copper used in electric wires comes in two flavors (isotopes): Cu-63 and Cu-65. Cu-63 has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, Cu-65, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu is 64.93 amu.
To learn more about average atomic mass here
https://brainly.com/question/28295541
#SPJ1
A gas fills a 22.4 L container and it is sealed when the gas measures being at STP. How many moles are in the container
Answers
At STP, a 22.4 L container contains 1 mole of gas according to the ideal gas law.
How many moles of gas are in a 22.4 L container at STP?At standard temperature and pressure (STP), the relationship between the volume of a gas and the number of moles it contains can be expressed as:
V = nRT/P
Where:
V = volume of gas (in liters)
n = number of moles of gas
R = ideal gas constant (0.08206 L·atm/mol·K)
T = temperature (in Kelvin, which is 273.15 + degrees Celsius)
P = pressure (in atmospheres)
At STP, T = 273.15 K and P = 1 atm. Substituting these values into the equation gives:
22.4 L = n (0.08206 L·atm/mol·K) (273.15 K) / 1 atm
Solving for n gives:
n = 1 mole
Learn more about Standard temperature and pressure
brainly.com/question/29129606
#SPJ11
TRUE/FALSE. most commonly known for its ability to bind to oxygen, iron also plays the important role of acting as a to many enzymes in numerous oxidation-reduction reactions.
Answers
Cofactor most commonly known for its ability to bind to oxygen, iron also plays the important role of acting as a to many enzymes in numerous oxidation-reduction reactions.
What does the chemical term "reaction" mean?Chemical processes occur when atoms form or break chemical bonds. The molecules that start a chemical are known as reactants, and the compounds which are generated as the result of reaction are known as products.
A physical reaction is what?A physical response is a reaction that modifies the physical characteristics of an item or material Physical characteristics encompass items like mass, volume, etc density.
To know more about Reaction visit:
https://brainly.com/question/28984750
#SPJ4