When 6.64 g of an unknown non-electrolyte is dissolved in 50.0 g of acetone,the boiling point increased to 57.3 degrees C from 56.2 degrees C. If the Kbp of the solvent is 1.71 K/m, calculate the molar mass of the unknown solute.
Answers
When 6.64 g of an unknown non-electrolyte is dissolved in 50.0 g of acetone, the boiling point increased to 57.3 degrees C from 56.2 degrees C. If the \(K_b_p\) of the solvent is 1.71 K/m, the molar mass of the unknown solute is 14.0 g/mol.
To calculate the molar mass of the unknown solute, we can use the equation for boiling point elevation:
ΔT = \(K_b_p\) * m * i
Where:
ΔT = Change in boiling point
\(K_b_p\) = Boiling point elevation constant for the solvent (in this case, acetone)
m = Molality of the solute (moles of solute per kilogram of solvent)
i = Van't Hoff factor (the number of particles the solute dissociates into in solution)
In this case, the unknown solute is a non-electrolyte, which means it does not dissociate into ions in solution. Therefore, the Van't Hoff factor (i) for the solute is 1.
Calculate the molality (m) of the solution:
Mass of acetone = 50.0 g
Molar mass of acetone = 58.08 g/mol
Moles of acetone = Mass / Molar mass = 50.0 g / 58.08 g/mol
Molality (m) = Moles of solute / Mass of solvent (in kg)
Molality (m) = (6.6 g / Molar mass of solute) / (50.0 g / 1000 g/kg)
m = (0.132 / Molar mass of solute)
Now, let's calculate the change in boiling point (ΔT):
ΔT = 59.9°C - 56.2°C = 3.7°C
Finally, Calculate the molar mass of the unknown solute by rearranging the equation and substituting the known values:
Molar mass of solute = (0.132 / (ΔT * \(K_b_p\)))
Molar mass of solute = (0.132 / (3.7 * 1.71))
Molar mass of solute ≈ 14.0 g/mol
Therefore, the molar mass of the unknown solute is approximately 14.0 g/mol.
To know more about molar mass here
https://brainly.com/question/30640134
#SPJ4
Related Questions
Succinic acid dissociates as follows: H₂C4H4O4+H₂O HC4H4O4 + H3O+ Ka1 = 6.2 × 10-5 HC4H4O4+H₂0 C4H4O42- + H3O+ Ka2 = 2.3 x 10-6 Calculate Kb1 and Kb2 for the following reactions (3 points): C4H4042 + H2O HC4H4O4 + OH- Kb1 = ? HC4H4O4+H₂O H2C4H4O4 + OH- Kb2 = ?
Answers
Kb1 = 1.61 × 10^-10 and Kb2 = 4.35 × 10^-9. The basicity constant, or Kb, is a measure of the strength of a base in a particular chemical reaction. The products of a reaction of a weak base and water with the corresponding acid determine the base constant.
For example, for a given acid and base, Kb1 and Kb2 are the basicity constants for the first and second base dissociations, respectively, of the base. The formulas and charges of the conjugate acid and base, as well as the acid dissociation constants, Ka1 and Ka2, are needed to calculate Kb1 and Kb2.
The following reactions are balanced chemical reactions that represent the dissociation of succinic acid:
Reaction 1: H2C4H4O4(aq) + H2O(l) ⇌ HC4H4O4(aq) + H3O+(aq) Ka1 = 6.2 × 10−5
Reaction 2: HC4H4O4(aq) + H2O(l) ⇌ C4H4O42-(aq) + H3O+(aq) Ka2 = 2.3 × 10−6
The values of Ka1 and Ka2 can be used to calculate Kb1 and Kb2, respectively, using the following equation:
Ka1 × Kb1 = Kw
where Kw is the ion-product constant for water, which is 1.0 × 10−14 at 25°C.
Kb1 can be calculated as follows:
Kw = Ka1 × Kb1
Kb1 = Kw / Ka1
Kw = 1.0 × 10^-14
Ka1 = 6.2 × 10^-5
Kb1 = Kw / Ka1
Kb1 = 1.0 × 10^-14 / 6.2 × 10^-5
Kb1 = 1.61 × 10^-10
Kb2 can be calculated using the same method:
Kw = Ka2 × Kb2
Kb2 = Kw / Ka2
Kw = 1.0 × 10^-14
Ka2 = 2.3 × 10^-6
Kb2 = Kw / Ka2
Kb2 = 1.0 × 10^-14 / 2.3 × 10^-6
Kb2 = 4.35 × 10^-9
Therefore, Kb1 = 1.61 × 10^-10 and Kb2 = 4.35 × 10^-9.
To learn more about basic visit;
https://brainly.com/question/31939284
#SPJ11
a chemistry student needs 85.0 g of dimethyl sulfoxide for an experiment. by consulting the crc handbook of chemistry and physics, the student discovers that the density of dimethyl sulfoxide is 1.10 g*cm^-3. calculate the volume of dimethyl sulfoxide the student should pour out. round your answer to significant digits.
Answers
The volume of Dimethyl sulfoxide the student should pour out is, 77.27ml
Since the 1960s, dimethyl sulfoxide (DMSO) has been used in humans for medical treatment and as a pharmacological agent. DMSO is now primarily used in the cryopreservation of stem cells, the treatment of interstitial cystitis, and as a penetrating vehicle for various drugs.
Dimethyl sulfoxide has a density of 1.10 g/103L, or 1.10 g/mL.
Now we develop the following argument:
If a volume of 1 mL is contained in 1.10 g of dimethyl sulfoxide
The volume of 85 g of dimethyl sulfoxide is then X mL.
Dimethyl sulfoxide: X = (85X1) / 1.10= 77.27ml
To know more about density visit
https://brainly.com/question/29775886
#SPJ4
Some students in a chemistry lab conducted an investigation in which they added four different solid substances to separate beakers of water. They stirred the mixtures for one minute and then recorded their observations in the table below. Which substance most likely caused a new substance to be formed when mixed with water?
1. The substance dissolved
2. The substance caused bubbles to form
3. The substance sank to the bottom
4. The substance floated to the top
Answers
A new substance to be formed when mixed with water if the substance caused bubbles to form.
What is a chemical reaction?A chemical reaction or a chemical change occurs when a new substance is formed. This could be signaled by an evolution of gas or the appearance of a solid.
In this case, a new substance to be formed when mixed with water if the substance caused bubbles to form.
Learn more about chemical reaction:https://brainly.com/question/8832859
#SPJ2
you have 100.0ml of 3.0M solution of ammonium hydroxide and 30.0g of potassium aluminum sulfate
a. What is the limiting reactant
b. What is the theoretical yield of aluminum hydroxide
C. could you collect above in a filter paper
Please show work/explain why
Answers
The limiting reactant is potassium aluminum sulfate.
To determine the limiting reactant, we need to compare the amount of each reactant to the stoichiometry of the balanced chemical equation. The balanced equation for the reaction between ammonium hydroxide (NH4OH) and potassium aluminum sulfate (KAl(SO4)2) is:
2 NH4OH + KAl(SO4)2 -> Al(OH)3 + (NH4)2SO4 + K2SO4
From the equation, we can see that the stoichiometric ratio between ammonium hydroxide and potassium aluminum sulfate is 2:1. Therefore, we need twice as many moles of ammonium hydroxide as potassium aluminum sulfate.
To calculate the moles of each reactant, we use the formula:
moles = concentration (M) × volume (L)
For the ammonium hydroxide:
moles of NH4OH = 3.0 M × 0.100 L = 0.300 mol
For the potassium aluminum sulfate:
moles of KAl(SO4)2 = mass (g) / molar mass (g/mol)
moles of KAl(SO4)2 = 30.0 g / (39.1 g/mol + 26.98 g/mol + 2(32.1 g/mol) + 4(16.0 g/mol)) = 0.083 mol
Since the stoichiometric ratio is 2:1, the moles of ammonium hydroxide are in excess.
To determine the theoretical yield of aluminum hydroxide (Al(OH)3), we need to convert the moles of the limiting reactant (potassium aluminum sulfate) to moles of the product using the stoichiometry of the balanced equation. From the balanced equation, we can see that the stoichiometric ratio between potassium aluminum sulfate and aluminum hydroxide is 1:1.The moles of aluminum hydroxide produced will be the same as the moles of potassium aluminum sulfate used, which is 0.083 mol.
To calculate the theoretical yield in grams, we use the formula:
mass = moles × molar mass
The molar mass of aluminum hydroxide (Al(OH)3) is (26.98 g/mol + 3(16.0 g/mol)) = 78.0 g/mol.
The theoretical yield of aluminum hydroxide is:
mass = 0.083 mol × 78.0 g/mol = 6.474 g
Therefore, the theoretical yield of aluminum hydroxide is 6.474 grams.
Aluminum hydroxide is a precipitate, which means it forms solid particles when the reaction occurs. It can be collected on a filter paper using a filtration process. Filtration is a common method to separate solids from liquids. The reaction mixture can be poured through a filter paper funnel, and the solid aluminum hydroxide particles will be trapped on the filter paper while the liquid and soluble salts (such as ammonium sulfate and potassium sulfate) pass through.However, it's important to note that the success of the filtration process depends on the particle size and the nature of the solid precipitate. If the particles of aluminum hydroxide are too fine or colloidal in nature, they may pass through the filter paper and affect the efficiency of the filtration. In such cases, additional techniques like centrifugation or using a finer filter may be required to achieve better separation.
Overall, collecting aluminum hydroxide on a filter paper is a feasible method in this scenario, provided the precipitate is of the appropriate size and nature for filtration.
for such more questions on reactant
https://brainly.com/question/26283409
#SPJ8
Which types of atomic orbitals of the central atom mix to form hybrid orbitals in:
(d) NF₃?
Answers
Hybrid orbitals form on the central atom and can either be sp, sp2, sp3, sp3d, or sp3d2. The type of hybrid orbitals that are used is dictated by the electron domain geometry.
Sigma bonds are formed by the end-to-end overlap of bonding orbitals. Pi bonds are formed by the side-to-side overlap of p orbitals.
How are hybrid orbitals formed?Hybrid orbitals are the result of a model which combines atomic orbitals on a single atom in ways that lead to a new set of orbitals that have geometries appropariate to form bonds in the directions predicted by the VSEPR model. The VSEPR model predicts geometries that are very close to those seen in real molecules.
What is the hybridization of nitrogen in NF3?Nitrogen trifluoride (NF3) lewis structure contains three sigma bonds and one lone pair around nitrogen atom. Therefore, there are total of four electrons regions around nitrogen atom. So, hybridization of center atom, nitrogen is sp3.
Learn more about NF3 here:
https://brainly.com/question/15351545#SPJ4What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
How many total atoms found in 2AClCl3
Answers
Answer: 4 atoms.
Explanation:
if ph was to decrease, while pco2 remained the same, how would [hco3-] and [co32-] change in seawater? which one would show the greater change? (explain your reasoning.)
Answers
If the pH of seawater decreases while pCO2 remains the same, the equilibrium between CO2, HCO3-, and CO32- will shift to compensate for the change in pH. Specifically, as pH decreases, the concentration of H+ ions increases, which will drive the reaction towards the consumption of H+ ions by HCO3- and CO32- to form carbonic acid (H2CO3):
CO2 + H2O <-> H2CO3 <-> H+ + HCO3- <-> 2H+ + CO32-
This will result in an increase in the concentrations of both HCO3- and CO32- in seawater. However, since the equilibrium constant (K) for the reaction HCO3- <-> H+ + CO32- is relatively small, the concentration of CO32- will change more than HCO3- in response to a change in pH. This is because any increase in H+ ions in the seawater will preferentially react with HCO3- to form H2CO3, which then drives the reaction to consume more CO32- to maintain the equilibrium.
Therefore, the concentration of CO32- will show a greater change than HCO3- in response to a decrease in pH, even though the concentration of both ions will increase.
What kind of reactions are redox reactions
Answers
A reaction in which reduction and oxidation take place simultaneously known as a redox reaction.
The heat of reaction for a chemical reaction can be calculated by finding the sum of the bond energies of the products and subtracting that from the sum of the bond energies of the reactants: Heat of reaction==Sum of the energy for the bonds broken − Sum of the energy for the bonds formedSum of reactant bond energies − Sum of product bond energies When calculating the sum of the bond energies, each bond in the reaction must be accounted for. For example, CH4 is a reagent with a coefficient of 1 in the reaction. There are four C−H bonds in methane and one methane molecule per reaction, for a total of four C−H bonds on the reactant side. All four bonds must be accounted for when finding the sum of the bond energies for the reactants. Calculate the heat of reaction using the average bond dissociation energies given in the introduction and your answer to Part B for the reaction CH4 + 2O2 → CO2 + 2H2O Express your answer in kilojoules per mole to three significant figures.
Answers
The heat of reaction = (sum of energy released in products) - (sum of energy required in reactants)
= 3454 kJ/mol - 2642 kJ/mol
= 812 kJ/mol
The heat of reaction for a chemical reaction can be calculated by finding the sum of the bond energies of the products and subtracting that from the sum of the bond energies of the reactants:
The heat of reaction = Sum of the energy for the bonds broken − Sum of the energy for the bonds formed
= Sum of reactant bond energies − Sum of product bond energies
When calculating the sum of the bond energies, each bond in the reaction must be accounted for.
\(CH_{4} + 2O_{2} → CO_{2} + 2H_{2} O\)
Reactants:
1 mole of CH4 has 4 C-H bonds, each with an average bond dissociation energy of 413 kJ/mol, so the total energy required to break these bonds is 4 x 413 kJ/mol = 1652 kJ/mol.
2 moles of O2 have 2 O=O bonds, each with an average bond dissociation energy of 495 kJ/mol, so the total energy required to break these bonds is 2 x 495 kJ/mol = 990 kJ/mol.
Therefore, the total energy required to break the bonds in the reactants is 1652 kJ/mol + 990 kJ/mol = 2642 kJ/mol.
Products:
1 mole of \(CO_{2}\) has 2 C=O bonds, each with an average bond dissociation energy of 799 kJ/mol, so the total energy released by the formation of these bonds is 2 x 799 kJ/mol = 1598 kJ/mol.
2 moles of \(H_{2}O\) have 2 O-H bonds and 2 H-O bonds, each with an average bond dissociation energy of 464 kJ/mol, so the total energy released by the formation of these bonds is 2 x (2 x 464 kJ/mol) = 1856 kJ/mol.
Therefore, the total energy released by the formation of the bonds in the products is 1598 kJ/mol + 1856 kJ/mol = 3454 kJ/mol.
Now we can calculate the heat of the reaction by subtracting the energy required to break the bonds in the reactants from the energy released by the formation of the bonds in the products:
The heat of reaction = (sum of energy released in products) - (sum of energy required in reactants)
= 3454 kJ/mol - 2642 kJ/mol
= 812 kJ/mol
Therefore, the heat of reaction for the given reaction is 812 kJ/mol to three significant figures.
Learn more about bond energy:
https://brainly.com/question/29151701
#SPJ11
Why is the atomic radius trend related to the electronegativity trend?
Answers
Here is the Answer I Hope this Helps:-
The closer the electrons are to the nucleus, the more tightly they are bound, thus increasing the electronegativity of the atom.
A student reads the statement shown.
"The force of gravity exerted on an object decreases as the distance between the objects increases."
Using this statement, what inference can the student make regarding the movement of the planets around the Sun?
A. The orbital speeds of larger planets will be faster than smaller planets.
B. The orbital speeds of gaseous planets will be faster than rocky planets.
C. The orbital speeds of planets closer to the Sun will be faster than planets farther away.
D. The orbital speeds of planets with smoother surfaces will be faster than planets with rougher surfaces.
Answers
Answer: C. The orbital speeds of planets closer to the Sun will be faster than planets farther away.
Explanation:
Centripetal force = gravitational force
\(\tt m\dfrac{v^2}{r}=Fg\)
\(\tt Fg=G\dfrac{M.m}{r^2}\)
Fg inversely proportional to distances (r)
\(\tt v^2=\dfrac{Fg.r}{m}\)
because the value of the gravitational force is getting smaller as the distance is getting further from the sun, the orbital speed of the planet will be getting smaller
A particular gas exerts a pressure of 3.38 bar. What is this pressure in units of atmospheres? (1 atm = 760 mm Hg = 101.3 kPa = 1.013 bar) Select one: a. 3.42 atm b. 2.54 × 103 atm c. 3.38 atm d. 2.6 × 103 atm e. 3.33 atm
Answers
To convert the pressure of a particular gas, expressed in bar units, to units of atmosphere, it is necessary to divide the given pressure by the value of one atmosphere in bar units. Thus, to convert 3.38 bar to atmospheres, it is necessary to divide 3.38 by 1.01325 bar/1 atm.
Pressure can be expressed in various units. One of the most commonly used units of pressure is the atmosphere, abbreviated atm. Other commonly used units of pressure include the torr, millimeters of mercury (mm Hg), kilopascals (kPa), and pounds per square inch (psi). To convert pressure from one unit to another, it is necessary to use conversion factors that relate the two units.
Here are some of the most commonly used conversion factors:1 atm = 760 mm Hg1 atm = 101.3 kPa1 atm = 1.01325 barTo convert a pressure expressed in one unit to another unit, it is necessary to use the appropriate conversion factor in a way that cancels out the initial unit and leaves the desired unit. For example, to convert 3.38 bar to atmospheres, it is necessary to use the conversion factor that relates bar to atmospheres:1 atm = 1.01325 barThis means that one atmosphere is equivalent to 1.01325 bar.
To know more about particular gas visit:
https://brainly.com/question/29803823
#SPJ11
predict the β-elimination product formed when each chloroalkane is treated with sodium ethoxide in ethanol. if two or more products might be formed, draw only the major product.
Answers
The major product for each reaction will depend on the stability of the alkene formed.
When each chloroalkane is treated with sodium ethoxide in ethanol, the β-elimination product that is formed can be predicted based on the size of the alkyl group and the position of the chlorine atom.
1-chloropropane: The reaction will form propene (C₃H₆) and sodium chloride (NaCl) as a byproduct.
2-chloropropane: The reaction will form propene (C₃H₆) and sodium chloride (NaCl) as a byproduct.
1-chlorobutane: The reaction will form 1-butene (C₄H₈) and sodium chloride (NaCl) as a byproduct.
2-chlorobutane: The reaction will form 1-butene (C₄H₈) and sodium chloride (NaCl) as a byproduct.
2-methyl-2-chloropropane: The reaction will form 2-methylpropene (C₄H₈) and sodium chloride (NaCl) as a byproduct.
2-chloro-2-methylbutane: The reaction will form 2-methylbutene (C₅H₁₀) and sodium chloride (NaCl) as a byproduct.
1-chloro-2-methylbutane: The reaction will form 2-methylbutene (C₅H₁₀) and sodium chloride (NaCl) as a byproduct.
The major product for each reaction will depend on the stability of the alkene formed. If more than one product is formed, the most stable alkene will be the major product.
know more about β-elimination product here: https://brainly.com/question/32799897
#SPJ11
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
If a person has type A– blood, then they have a) only the A protein b) both the A and the Rh proteins c) all three blood proteins d) It is impossible to tell what proteins they have
Answers
If a person has type A– blood, then they have only the A protein in blood. Therefore, option A is correct.
What is protein ?A structure composed of amino acids. The body need proteins to function properly. They serve as the building blocks for several bodily components, including the skin, hair, and enzymes, cytokines, and antibodies.
Large, intricate molecules known as proteins play a variety of vital functions in the body. They are crucial for the structure, operation, and control of the body's tissues and organs and carry out the majority of their job inside cells.
A+ blood indicates that your red blood cells contain both the A antigen and the Rhesus factor. On the other hand, if you have type A- blood, all the blood cells carry the A antigen.
Thus, option A is correct.
To learn more about protein, follow the link;
https://brainly.com/question/2193769
#SPJ1
define volume
-length x width x height
-the mass of liquid required to fill a container
-the space occupied by a liquid
-the space occupied by a quantity of matter
Answers
the space occupied by quantity of matter
Why is fluorine more reactive than and bromine
Answers
Answer:
- The Valence/Bonding Electrons (whatever name you address them as) in Flourine are closer to the nucleus than the Valence/Bonding Electrons are in an atom of Bromine.
Explanation:
Hopefully this was helpful!
What is the ratio of [h 2po 4 -] to [hpo 4 2-] at ph 6.4 if the pka of h 2po 4 - is 7.4?
Answers
The Henderson-Hasselbalch equation allows us to determine the relative concentrations of an acid and its conjugate base based on the pH of the solution and the pKa of the acid. At pH 6.4, the ratio is 0.1.
The Henderson-Hasselbalch equation is a mathematical relationship that relates the pH of a solution to the ratio of the concentration of an acid and its conjugate base. It is commonly used in chemistry and biochemistry to describe the acid-base equilibrium.
To determine the ratio of \([H-2PO_4-]\) to \([HPO_4^2-]\) at pH 6.4, we can use the Henderson-Hasselbalch equation:
\(pH = pKa + log([A-]/[HA])\)
In this case, \(H_2PO_4-\) is the acid (HA) and \(HPO_4^2-\) is its conjugate base (A-).
Given that the pKa of \(H_2PO_4-\) is 7.4, we can substitute the values into the equation:
\(6.4 = 7.4 + log([HPO_4^2-]/[H_2PO_4-])\)
Simplifying the equation:
\(-1 = log([HPO_4^2-]/[H_2PO_4-])\\[HPO_4^2-]/[H_2PO_4-] = 10^{(-1)} = 0.1\)
Therefore, at pH 6.4, the ratio is 0.1.
For more details regarding the Henderson-Hasselbalch equation, visit:
https://brainly.com/question/31495136
#SPJ4
3. The picture on the right is an example of.

Answers
Accuracy and precision is what the picture on the right is an example of.
Why accuracy and precision?Accuracy and precision are the two main concepts represented in the Venn diagram. Accuracy refers to how close a measurement is to the true value, while precision refers to how close repeated measurements are to each other.
In the Venn diagram, the area of overlap represents the measurements that are both accurate and precise. The area outside of the circles represents the measurements that are either inaccurate or imprecise.
For example, if you are measuring the length of a table, an accurate measurement would be one that is very close to the actual length of the table. A precise measurement would be one that is repeated multiple times and the results are very close to each other.
Find out more on accuracy and precision here: https://brainly.com/question/1695072
#SPJ1
Assignment (5)
(1) It takes 40 mL of a 0.25 M NaOH solution to neutralize 100
mL of an HCl solution. What is the concentration of the HCI
solution?
(2) It takes 72 mL of 0.65M NaOH solution to completely
neutralize 125 ml of a sulfuric acid solution (H2SO4). What
is the concentration of the H2SO4 solution?
Answers
a) The concentration of the HCl solution is 0.1 M.
b) The concentration of the H₂SO₄ solution is 0.1872 M.
a) To solve both of these problems, we can use the formula M₁V₁ = M₂V₂, where M₁ is the concentration of the first solution, V₁ is the volume of the first solution used, M₂ is the concentration of the second solution, and V₂ is the volume of the second solution used.
For the first problem, we are given that 40 mL of a 0.25 M NaOH solution neutralizes 100 mL of an HCl solution. We can use the formula to solve for the concentration of the HCl solution:
0.25 M x 40 mL = M₂ x 100 mL
M₂ = (0.25 M x 40 mL) / 100 mL
M₂ = 0.1 M
b) For the second problem, we are given that 72 mL of a 0.65 M NaOH solution neutralizes 125 mL of an H₂SO₄ solution. However, H₂SO₄ is a diprotic acid, meaning that it can donate two hydrogen ions (H⁺) per molecule.
Therefore, we need to account for this when calculating the concentration of the H₂SO₄ solution:
0.65 M x 72 mL = 2 x M₂ x 125 mL
M₂ = (0.65 M x 72 mL) / (2 x 125 mL)
M₂ = 0.1872 M
To learn more about concentration click on,
https://brainly.com/question/3045247
#SPJ4
the method of mass production that developed during the nineteenth century was a process that
Answers
Answer:
Relied on the use of power driven machinery
Explanation:
Its because the machines would increase the mass productivity of goods
consider carbonyl compounds a-e drawn below. in this question you will rank these compounds in order of stability and reactivity. part 1 out of 4 rank a-e in order of increasing stability. (you should consider the stability of the carbonyl functional group.) smith6e1853 which of the following options correctly places these compounds in order of increasing stability? b < d < a < e < c a < e < c < d < b c < e < a < d < b a < c < e < d < b e < c < a < d < b
Answers
The order of increasing stability of the carbonyl functional group is e < c < a < d < b..
What is carbonyl ?Carbonyl is an organic compound that contains a carbon-oxygen double bond (C=O). This double bond is one of the most important functional groups in organic chemistry, as it exists in a variety of compounds and can undergo a wide range of reactions. The carbonyl group is composed of a carbon atom bonded to an oxygen atom that is doubly bonded to the carbon atom. This double bond gives the carbonyl group special reactivity, as the electrons in the double bond can be used to form new bonds with other atoms.
This is because the compounds with the most electron-withdrawing groups on the carbonyl carbon are the most stable. Compound e is the most stable, as it has a triple bond on the carbonyl carbon. Compound c is the next most stable, as it has a halogen (Cl) substituent on the carbonyl carbon. Compound a is the third most stable, as it has an ether group on the carbonyl carbon. Compound d is the fourth most stable, as it has an alkyl group on the carbonyl carbon. Finally, compound b is the least stable, as it has no substituents on the carbonyl carbon.The correct answer is e < c < a < d < b .
To learn more about Carbonyl
https://brainly.com/question/13564853
#SPJ4
What are the types of precipitation?
Select four correct answers.
(1) dew
(2) hail
(3) wind
(4) rain
(5) sleet
(6) fog
(7) snow
(I might answer it by myself so be quick)
Answers
Answer:
Rain,Hail,Snow,sleet. Hope this helped you out!
Problem: Co3+ | Co2+ and Ni2+ | NiAnode?Cathode?(You need to use Reference Table B-16.)a. Co2+b. can't answerc. Ni2+d. Nie. Co3+

Answers
Answer:
- Anode: Co3+ | Co2+
- Cathode: Ni | Ni2+
Explanation:
The anode is where oxidation reaction occurs, and the cathode is where reduction reaction occurs.
From the table of reduction potencials, we find that:
- Co reaction:
\(\begin{gathered} Co^{3+}+2e^-\rightarrow Co^{2+} \\ E=1.81\text{ }V \end{gathered}\)- Ni reaction:
\(\begin{gathered} Ni\rightarrow Ni^{2+}+2e^- \\ E=-0.250\text{ V} \end{gathered}\)Now, to find out which one is the anode and which one is the cathode, it is necessary to compare the reduction potencials.
The reaction of Ni have negative potentials, so Ni will be the anode and Co will be the cathode.
11.Three atoms of element X react with an element(s) Y from group VIA. What
would be the chemical formula between X and Y.
Answers
Element “X” belongs to family 2A, meaning it is part of the family that includes elements such as Be and Mg. These elements will all have 2 valence electrons and can be represented by the following Lewis diagram:
Element “Y” belongs to family 5A, meaning it is part of the family of elements that includes elements such as N and P. These elements will all have 5 valence electrons and can be represented by the following Lewis diagram:
Atoms from these two families will usually react with each other by losing or gaining valence electrons to create stable ions (an ion is what we call an atom/particle that has a charge). These stable ions form by the atoms either losing or gaining electrons until they have the same number of valence electrons as the nearest Noble Gas. This means that each ion will have a full valence shell (usually consisting of 8 electrons), often referred to as a “stable octet”, and this process of creating stable ions is often called the “octet rule”.
Atoms with fewer that 4 valence electrons will normally have a weak hold on their valence electrons and will tend to lose their valence electrons when forming ions.
Atoms with 4 or more valence electrons will normally have a strong hold on their valence electrons and will tend to gain electrons when forming ions.
The charge on the ion arises from the fact that, initially, the atom is electrically neutral because it has the same number of electrons (negative charges) as protons (positive charges). By losing electrons, the atom will end up with more protons (positive charges) than electrons (negative charges) and will form an ion with an overall positive charge. By gaining electrons, the atom will end up with more negative electrons than positive protons becoming an ion with an overall negative charge.
So, an atom of element “X”, with only 2 valence electrons, must lose its 2 valence electrons (which will be gained by element “Y”) to form a stable ion with a 2+ charge (losing two electrons leaves the ion with 2 more positive charges (protons) than negative charges, so a net charge of 2+).
An atom of element “Y”, with 5 valence electrons, must gain 3 electrons (from element “X”) to form a stable ion with a 3- charge (gains 3 extra negative charges).
We can show this process using Lewis diagrams:
From this set of diagrams you can see that in order to create stable ions of both “X” and “Y” we need these atoms to react with each other in a 3:2 ratio (we need 3 atoms of X for every 2 atoms of Y). This means that the resulting chemical formula of the compound will be:
Now, we will look at a short cut that can help you figure this out without having to draw Lewis diagrams.
Compounds are electrically neutral, meaning they must contain equal numbers of positive and negative charges. For compounds consisting of oppositely charged ions, this means that the total charge of the negative ions must be equal to the total charge of the positive ions. In other words the ions must combine in a ratio that makes their charges add to zero.
If we look at the compound we just made, X3Y2, we can confirm this:
So, now you can you predict the formula of simple ionic compounds:
from the family of elements, determine the number of valence electrons each element has
determine the charge of the ions that each atom will form using the octet rule (or look on the periodic table, most will tell you the stable ionic charges that each element can form)
determine the ratio of positive ions to negative ions that results in an overall charge of zero
Example,
What is the formula of a compound produced when an element from family 3A combines with an element from family 7A?




Explain how Coulomb's law can help you understand why some interparticle forces are stronger than others.

Answers
Answer:
Coulombs equation involves distance and the distance from one radius to the next allows us to predict certain characteristics such as pull and attraction. For example, you would expect, a higher charge on the ion and a higher dipole moment causes a larger force. This is due to the magnitude which is the distance between these two and effectively that is how Columbus law comes into play.
Coulomb's law states that the force of repulsion or attraction among both two charged bodies is directly proportional to the product of their charges as well as inversely proportional to the square of the length between them.
What is Coulomb's law?An electric force is created when two charged particles collide. The forces will be greater if you have larger charges.
Coulomb's Law can be understood by combining these two ideas and the fact that charges can attract and repel each other. It is a formula for calculating the electrical forces between two objects.
Coulomb's equation involves distance, and the distance between two radiuses allows us to predict certain characteristics such as pull and attraction.
As you might expect, a higher charge on the ion and a higher dipole moment result in a greater force. This is due to the magnitude of the distance between these two, and this is where Columbus law comes into play.
Thus, this way, Coulomb's law can help you understand why some interparticle forces are stronger than others.
For more details regarding Coulomb's law, visit:
https://brainly.com/question/506926
#SPJ2
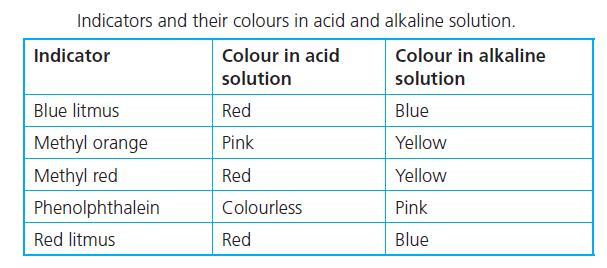
help i suck at chemistry

Answers
Answer:
1. Acid - Red
2. Base - Yellow
3. Salt - Yellow if the reaction produces a base
Explanation:
In an acidic medium, methyl orange turns red, while in a basic medium, it turns yellow.
Sodium chloride solution produces sodium hydroxide, NaOH which is a strong base. Using methyl orange as an indicator gives a yellow colour solution for NaOH.
There are acidic, neutral, and basic salts. Sodium chloride (NaCl) produces a base therefore it would turn yellow as well but likely less distinct than the base.
Answer:
Hello methyl orange is a pH indicator that is commonly used.
If you drip methyl orange to an acidic liquid it will give you the color red.
If it turns yellow after you drip it then the liquid should be a base.
And it gives a yellowish color for neutral liquids
But in this case salt (NaOH) has an exceptional situation which turns orange after adding m.o.
There is no logical explanation (at least for high school level) I am afraid that you need to memorize it.
This chard attached below may help you to recognize it
good luck, hope it helped<3!
chemical porperties of synthetic fiber
Answers
Explanation: Some of the most important properties of synthetic materials are as follows: 1. Tensile strength 2. The Action of water 3. The Action of heat and flame 4. Thermal conductivity 5. Electrical conductivity. The usefulness or otherwise of a synthetic material depends upon the following properties. 1. Tensile strength:
PLEASE MARK ME AS THE BRAINLIEST
The temperature of a 0.65L sample of carbon dioxide gas is 580K. If the pressure remains constant, what is the new volume of the gas if the temperature increases to 1300K?