When drawing the Lewis structure of the H,CO molecule, the structure should represent a total of 12 valence electrons. Based on the elements present, a total of electrons are needed for a stable structure. Thus, there should be bonds in the structure, The ____... - atom should be in the center with ____... and _____.
Answers
When drawing the Lewis structure of the H, CO molecule, the structure should represent a total of 12 valence electrons. The carbon atom should be in the center with one hydrogen and one oxygen.
What is Lewis structure?A Lewis structure is a diagram that shows the lone pairs and bonding pairs of electrons in a molecule or ion. Valence electrons are the outermost electrons of an atom that take part in chemical reactions. They are placed on the Lewis structure's outermost orbitals.
The Lewis dot structure of CO and H are given below: Carbon has four valence electrons, and oxygen has six valence electrons. Hydrogen has one valence electron. The total valence electrons for CO and H can be calculated as follows:
Valence electrons for CO: Valence electrons for C = 4
Valence electrons for O = 6
Total valence electrons for CO = 4 + 6 = 10
Valence electrons for H : Valence electrons for H = 1
Total valence electrons for H₂O = 1 × 2 = 2
Total valence electrons for H, CO = 10 + 2 = 12
In the Lewis structure of H, CO, the carbon atom should be in the center with one hydrogen and one oxygen. The carbon atom, which is the least electronegative element, should be in the center since it has to make the most bonds. One oxygen and one hydrogen atom should be bonded to the carbon atom. There should be one double bond between carbon and oxygen.
Learn more about Lewis structure here:
https://brainly.com/question/20300458
#SPJ11
Related Questions
Argon has a pressure of 34.6 atm. It is transferred to a new tank with a volume of 456 L and pressure of 2.94 atm. What was the volume of the original container?
Answers
Answer:
38.75 L
Explanation:
From the question,
Applying Boyles Law,
PV = P'V'....................... Equation 1
Where P = Original pressure of the Argon gas, V = Original Volume of Argon gas, P' = Final pressure of Argon gas, V' = Final Volume of Argon gas.
make V the subject of the equation
V = P'V'/P.................... Equation 2
Given: P = 34.6 atm, V' = 456 L, P' = 2.94 atm.
Substitute these values into equation 2
V = (456×2.94)/34.6
V = 38.75 L
How many molecules are in 1.2 L of CH4 at STP?
Answers
Answer: 4.48L of CH4 at STP is equal to 1.2×10^23 molecules
Explanation:
the inital processing of iron ore (fe2o3) is by reacting it with coke (essentially elemental carbon) to make fo and carbon dioxide.
Answers
If the reaction proceeds with a 100% yield, the mass of \(FeO\)produced will be 128.653 grams.
To write the balanced equation for the reaction between iron (Fe) and carbon (C) to form iron(II) oxide (\(FeO\)) and carbon dioxide (\(CO_2\)), we first need to determine the appropriate formulas for the compounds involved.
1. Iron: Fe
2. Carbon: C
3. Iron(II) oxide: \(FeO\)
4. Carbon dioxide: \(CO_2\)
The balanced equation for the reaction is as follows:
\(2Fe + C - > FeO + CO_2\)
Now, let's calculate the theoretical yield of \(FeO\) when starting with 200.0g of Fe and 40.0g of C, assuming a 100% yield.
First, we need to convert the given masses of Fe and C into moles using their respective molar masses.
Molar mass of Fe = 55.845 g/mol
Molar mass of C = 12.011 g/mol
Moles of Fe = mass of Fe / molar mass of Fe
= 200.0g / 55.845 g/mol
= 3.579 mol
Moles of C = mass of C / molar mass of C
= 40.0g / 12.011 g/mol
= 3.331 mol
From the balanced equation, we can see that the stoichiometric ratio between Fe and \(FeO\) is 2:1.
Therefore, the moles of \(FeO\) formed will be half the moles of Fe used.
Moles of \(FeO\) = (1/2) * moles of Fe
= (1/2) * 3.579 mol
= 1.790 mol
Now, we can calculate the mass of \(FeO\) using its molar mass.
Molar mass of \(FeO\) = 71.844 g/mol
Mass of \(FeO\) = moles of \(FeO\) * molar mass of \(FeO\)
= 1.790 mol * 71.844 g/mol
= 128.653 g
Therefore, if the reaction proceeds with a 100% yield, the mass of \(FeO\)produced will be 128.653 grams.
To learn more about mass ,
https://brainly.com/question/24191825
#SPJ4
The complete question is:
The initial processing of iron ore (\(Fe_2O_3\)) is by reacting it with coke (essentially elemental carbon) to make \(FeO\) and carbon dioxide. Write a balanced equation. begin with 200.0g of Fe; and 40.0g of carbon, what is the mass of \(FeO\) produced when the yield is 100%?
If 33.0 grams of hydrogen gas react with 300. grams of oxygen gas, what is the percent yield if an experiment produces 250. grams of water? Use the balanced equation from question 3.
Answers
Answer:
can you show us question 3 pls
Explanation:
How many molecules are in 48.0 grams of NaOH?
Answers
Hope this helped :)
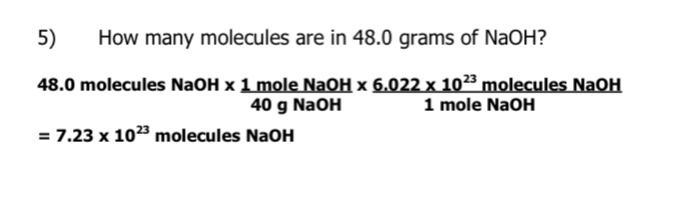
Calculate the frequency of a wave with a wavelength is 6.0 x 10-7 m.
Answers
The frequency of a wave with a wavelength of 6.0 x 10-⁷m is 5.0 × 10¹⁴Hz.
How to calculate frequency?The frequency of a wave can be calculated by dividing the wavelength by its wavelength as follows:
f = v/λ
Where;
f = frequencyv = speed of light (3 × 10⁸m/s)λ = wavelengthAccording to this question, a wave has a wavelength of 6.0 x 10-⁷m. The frequency can be calculated as follows:
f = 3 × 10⁸ ÷ 6.0 × 10-⁷
f = 5.0 × 10¹⁴Hz
Therefore, the frequency of a wave with a wavelength of 6.0 x 10-⁷m is 5.0 × 10¹⁴Hz.
Learn more about frequency at: https://brainly.com/question/14316711
#SPJ1
Yo I rly need help plzzz

Answers
Were the efforts to make quinine cheaper and more accessible an example of pure science or applied science? Explain.
Answers
Pure science deals with the process of discovering new scientific facts through carefully controlled scientific experiments while applied science deals with the use of scientific knowledge to solve problems in society. Efforts to make quinine cheaper and more accessible is an example of applied science.
The scientific efforts that are concentrated on making new facts available is known as pure science. Efforts such as discovery of new elements is an example of pure science.
The scientific efforts that are concentrated on applying scientific knowledge in solving new problems in the world is an example of applied science.
Efforts to make quinine cheaper and more accessible is an example of applied science.
Learn more: https://brainly.com/question/13675957
The image shows particles of salt dissolved in water.
How will the arrangement of salt particles most likely change?
Salt particles will move toward the left through diffusion.
Salt particles will move toward the left through active transport.
Salt particles will move toward the right through diffusion.
Salt particles will move toward the right through active transport.
Answers
Answer: Hope it may help you
Explanation:
Diffusion can be defined as the movement of particles from a region where there a more concentration of solute to the region having less concentration of the solute.
Here, the salt particles will move from right side of the box to the left side of the box.
This is because the right side has more particle and the left side has less particles so they will move towards the left side.
Answer:
A
Explanation:
Why is creativity Important and construction scientific methods?
I
Answers
Explanation:
Sometimes it is necessary to put forth new theories to explain experimental results, Explanation:
Creativity is important to science because sometimes, it is necessary to put forth new theories to explain experimental results. Theories are explanation of scientific observations.
Answer: Without creativity, it would be difficult to come up with new methods to answer scientific questions, meaning we likely wouldn't learn anything new.
Explanation: took the quiz
Calculate the standard potential for the following galvanic cell:
Ni(s) | Ni2+(aq) | Ag+(aq) | Ag(s)
which has the overall balanced equation:
Ni(s)+2Ag+(aq)→Ni2+(aq)+2Ag(s)
Express your answer to three significant figures and include the appropriate units.
Reduction half-reaction E∘ (V)
Ag+(aq)+e−→Ag(s) 0. 80
Cu2+(aq)+2e−→Cu(s) 0. 34
Ni2+(aq)+2e−→Ni(s) −0. 26
Fe2+(aq)+2e−→Fe(s) −0. 45
Zn2+(aq)+2e−→Zn(s) −0. 76
Answers
The standard potential for the given galvanic cell is +1.06 V.
To calculate the standard potential for the given galvanic cell, we need to determine the individual reduction potentials of the half-reactions and then subtract the potential of the anode (where oxidation occurs) from the potential of the cathode (where reduction occurs).
Given reduction half-reaction potentials:
Ag+(aq) + e^− → Ag(s): E∘ = +0.80 V
Ni2+(aq) + 2e^− → Ni(s): E∘ = -0.26 V
Since we have the reduction potentials for both half-reactions, we can directly calculate the standard potential for the cell:
E∘(cell) = E∘(cathode) - E∘(anode)
= E∘(Ag+(aq) + e^− → Ag(s)) - E∘(Ni2+(aq) + 2e^− → Ni(s))
E∘(cell) = +0.80 V - (-0.26 V)
= +1.06 V
Learn more about standard potential here
https://brainly.com/question/31868529
#SPJ11
What are the different states of matter and how do they transition from one to
the other? Help plz
Answers
Solid is the state in which matter maintains a fixed volume and shape; liquid is the state in which matter adapts to the shape of its container but varies only slightly in volume; and gas is the state in which matter expands to occupy the volume and shape of its container.??.....
Octane has a density of 0.702 g slash centimeters cubed what is the mass of 32 cm³ of octane
Answers
\(\\ \rm\longmapsto Density=\dfrac{Mass}{Volume}\)
\(\\ \rm\longmapsto Mass=Density\times Volume\)
\(\\ \rm\longmapsto Mass=0.702(32)\)
\(\\ \rm\longmapsto Mass=22.464g\)
Why Atomic size decreases as we go from left to right in Modern Periodic Table?
Answers
Answer:
There is an increase in nuclear charge.
who else had to do a final model for the rust unit
Answers
According to the bohr model of the atom, the single electron of a hydrogen atom circles the nucleus?
Answers
According to the bohr model of the atom, the single electron of a hydrogen atom circles the nucleus is specific allowed orbits.
What is orbits?Encyclopedic entry. An orbit is a regular, repeating path that one object takes around another object or center of gravity. Orbiting objects, which are called satellites, include planets, moons, asteroids, and manmade devices.An orbit is a regular, repeating path that one object takes around another object or center of gravity. Orbiting objects, which are called satellites, include planets, moons, asteroids, and manmade devices.Objects orbit each other because of gravity. Gravity is the force that exists between any two objects with mass. Every object, from the smallest subatomic particle to the largest star, has mass. The more massive the object, the larger its gravitational pull. Gravitational pull is the amount of force one object exerts on another object.
To learn more about Gravitational refer to:
https://brainly.com/question/27943482
#SPJ4
Technician A says that DOT 3 brake fluid has a higher boiling point than DOT 5.Technician B says that DOT 4 brake fluid has a lower boiling point than DOT 5. who is correct? a) a only b)b only c)both A and B
Answers
Technician A is incorrect, and Technician B is partially correct as DOT 4 has a lower boiling point than DOT 3.
Neither one of the experts is totally right. Dab 3 and Speck 4 brake liquids are glycol-based and have a higher limit than Dab 5 silicone-based brake liquid. In this manner, Specialist An is erroneous as Spot 5 has a higher edge of boiling over than Speck 3 and Dab 4 liquids.
Then again, Specialist B is wrong as Dab 4 brake liquid has a higher edge of boiling over than Spot 5 liquid, yet it is still lower than the limit of Speck 3 liquid. By and large, higher edge of boiling over brake liquid is alluring as it opposes disintegrating and brake disappointment under high-temperature conditions.
It means a lot to utilize the fitting kind of brake liquid determined by the vehicle maker to guarantee legitimate brake capability and security.
To learn more about brake fluid, refer:
https://brainly.com/question/29511747
#SPJ4
Explain the process of how James Chadwick
found the neutron.
Answers
Answer:
In 1932, the physicist James Chadwick conducted an experiment in which he bombarded Beryllium with alpha particles from the natural radioactive decay of Polonium. The resulting radiation showed high penetration through a lead shield, which could not be explained via the particles known at that time.
Explanation:
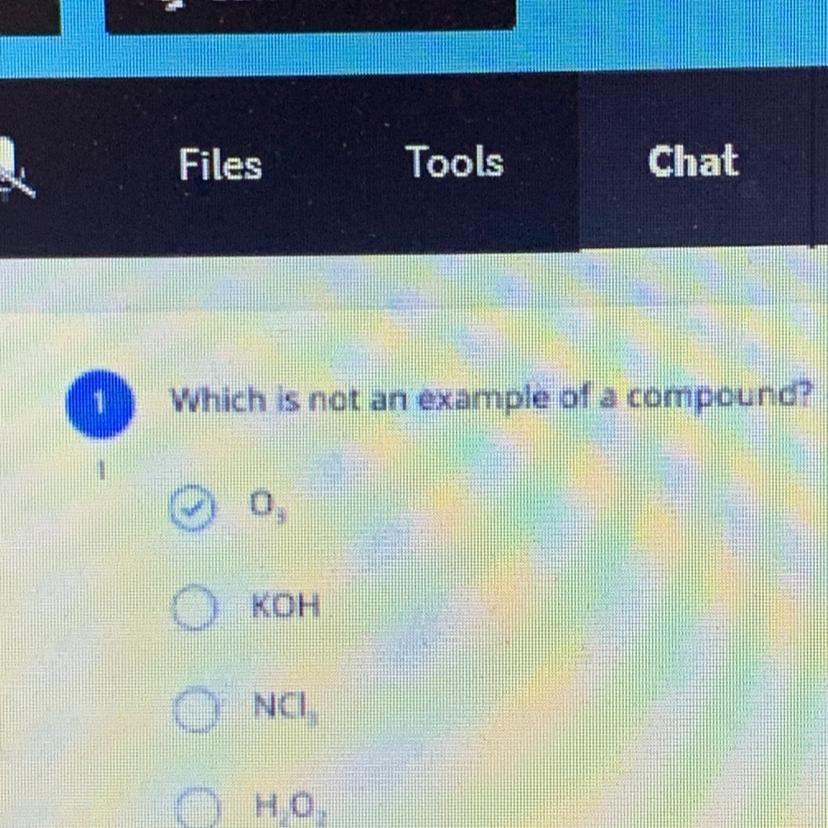
Which is not an example of a compound?
Ingnore the top PLEASE HELPP
Answers
Answer:
The 3rd answer or NCI is the correct answer
Explanation:
Hope this helps:)
Answer:
the 1st one
Explanation:
A voltaic cell is constructed in which the following cell reaction occurs. the half-cell compartments are connected by a salt bridge. ag (aq) cr2 (aq) ag(s) cr3 (aq)
Answers
The cell reaction is Ag(aq) + Cr3+(aq) → Ag(s) + Cr2+(aq). The standard cell potential is 0.742 V.
A voltaic cell is a device that utilizes a spontaneous chemical reaction to generate electricity. A half-cell is a component of the voltaic cell that contains either an oxidizing or reducing agent.
The salt bridge is used to connect the two half-cells and prevent mixing of the solutions.The given cell reaction is Ag(aq) + Cr3+(aq) → Ag(s) + Cr2+(aq).
This reaction takes place in two half-cells.
In one half-cell, Ag is oxidized to Ag+.
In the other half-cell, Cr3+ is reduced to Cr2+.
The cell potential, Ecell, can be calculated using the Nernst equation:
Ecell = E°cell - (RT/nF)
lnQwhere E°cell is the standard cell potential,
R is the gas constant,
T is temperature in kelvin,
n is the number of electrons transferred in the reaction,
F is the Faraday constant, and
Q is the reaction quotient.
The standard cell potential for the given cell reaction is 0.742 V.
Therefore, the cell generates 0.742 volts of electrical potential difference.
To know more about potential difference visit:
https://brainly.com/question/24142403
#SPJ11
The gas-phase reaction between nitrogen and oxygen was carried out in a device designed to maintain constant pressure. There are two cylinders of equal volume with a reaction arrow between them. The cylinder on the left has two molecules of O 2 and two molecules of N 2. The cylinder on the right has four molecules of N O. A constant pressure is applied to both cylinders Write the balanced chemical equation for the reaction between nitrogen oxygen. Include physical states. Predict wether the work for the reaction is positive or negative or zero. Using the date determine the enthalpy of the reaction for the formation of 1 mole


Answers
Answer:
The answer should be that a constant pressure is applied to both cylinders and they are balanced chemical equations for the reaction between nitrogen oxygen.
Explanation:
The unique aspect of a saturated hydrocarbon is that it must contain
A) only carbon and hydrogen.
B) only single bonds.
C) single and double bonds.
D) single, double and triple bonds.
Answers
The correct answer is A) only carbon and hydrogen.
A saturated hydrocarbon is a hydrocarbon compound consisting solely of carbon and hydrogen atoms, and it is characterized by having the maximum number of hydrogen atoms bonded to each carbon atom. In other words, the carbon atoms in a saturated hydrocarbon are fully "saturated" with hydrogen atoms, meaning that they form only single bonds with other atoms.
Hydrocarbons are organic compounds composed of hydrogen and carbon atoms. They are the fundamental building blocks of many important substances, including fossil fuels and various organic compounds found in living organisms. Hydrocarbons can be classified into two main types: saturated and unsaturated.
Saturated hydrocarbons, also known as alkanes, have a general molecular formula of CnH2n+2. They are characterized by the presence of only single covalent bonds between carbon atoms, forming a linear or branched chain structure. Because each carbon atom forms four single bonds, it is bonded to the maximum number of hydrogen atoms possible, hence the term "saturated." Examples of saturated hydrocarbons include methane (CH4), ethane (C2H6), and propane (C3H8).
On the other hand, unsaturated hydrocarbons contain at least one double or triple bond between carbon atoms. These bonds can be either double bonds (C=C) or triple bonds (C≡C) and allow for the possibility of additional atoms or functional groups to be attached to the carbon skeleton. Unsaturated hydrocarbons include alkenes and alkynes, and examples include ethene (C2H4) and ethyne (C2H2).
In conclusion, the unique aspect of a saturated hydrocarbon (option A) is that it must contain only carbon and hydrogen atoms, forming single bonds between carbon atoms. It does not include double or triple bonds found in unsaturated hydrocarbons (options B, C, and D).
Learn more about Saturated Hydrocarbons :
https://brainly.com/question/1364774
#SPJ11
True or false? The ground state electron configuration for manganese is [Ar]4 s
2
4 d
5
. True or false? The ground state electron configuration for calcium is 1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
. How many valences electron dose Si(z=14) continue? How many orbitals are in the 4 s sublevel? How can I calculate the values of JJ (total angular momentum) for a particular term, for instance,
3
P ?
Answers
The ground state electron configuration for manganese is False. The ground state electron configuration for calcium is True.
The ground state electron configuration for manganese is False. The correct ground state electron configuration for manganese (Z = 25) is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵
The ground state electron configuration for calcium is True. The correct ground state electron configuration for calcium (Z = 20) is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
For silicon (Z = 14), the electron configuration is:
1s² 2s² 2p⁶ 3s² 3p²
Therefore, silicon has 4 valence electrons.
The 4s sublevel can hold a maximum of 2 electrons. It consists of one orbital.
To calculate the values of JJ (total angular momentum) for a particular term, you need to consider the electron configuration and Hund's rule.
For the 3P term, the electron configuration would be:
3s² 3p³
To calculate the values of JJ, you need to consider the total number of electrons in the term. In this case, there are 5 electrons. According to Hund's rule, the maximum value of J is determined by the total number of unpaired electrons. Since there are 3 unpaired electrons in the 3P term, J can have values ranging from 3 - 1 to 3 + 1, which are 2 and 4. Therefore, for the 3P term, the possible values of JJ are 2 and 4.
To know more about electronic configuration:
https://brainly.com/question/31812229
#SPJ4
Classify each organic compound based on the functional group it contains.
ether
carboxylic acid
ester
alcohol
Molecular Structure
Type of Compound
I-O-I
Η Ο
1 10
C-C-0-H
1
H
H
-I
H
II
H
1
H-C
1
H
H
1
-C
1
H
1
H
1
H
1
H
H
I
H-C
1
H
H H
I
C-0-C-H
1
1
H H
1
H-CC-O-H
1
H H
Answers
Answer:
H2C2O1
Explanation:
Answer:
Carboxylic Acid
Ester
Ether
Alcohol
Explanation:

Carbon-14 has a half-life of 5,730 years. How long will it take for 112. 5 g of a 120. 0-g sample to decay radioactively? 5,730 years 11,460 years 17,190 years 22,920 years.
Answers
The time taken by Carbon-14 to decay radioactively from 120g to 112.5g is 22,920 years.
How do we calculate the total time of decay?Time required for the whole radioactive decay of any substance will be calculated by using the below link:
T = (n)(t), where
t = half life time = 5730 yearsn = number of half life required for the decayInitial mass of Carbon-14 = 120g
Final mass of Carbon-14 = 112.5g
Left mass = 120 - 112 = 7.5g
Number of required half life for this will be:
1: 120 → 602: 60 → 303: 30 → 154: 15 → 7.54 half lives are required, now on putting values we get
T = (4)(5730) = 22,920 years
Hence required time for the decay is 22,920 years.
To know more about radioactive decay, visit the below link:
https://brainly.com/question/24115447
#SPJ1
if you dissolve 93.1g of k2CO3(s) (molar mass=136.21 g/mol) in enough water to produce a solution with a volume of 1.09 L. what is the molarity
Answers
Answer: The molarity of the K2CO3 solution is 0.625 M.
Explanation: To find the molarity of a solution, you need to know the moles of solute and the volume of the solution in liters. Here's how to solve the problem:
Calculate the moles of K2CO3 using its given mass and molar mass:
moles = mass / molar mass = 93.1 g / 136.21 g/mol = 0.682 mol
Calculate the volume of the solution in liters:
volume = 1.09 L
Calculate the molarity of the solution using the moles and volume:
molarity = moles / volume = 0.682 mol / 1.09 L = 0.625 M
On which part of an sds would you find information about the symptoms and effects of exposure to the chemical substance?
Answers
Blindness is part of an SDS would you find information about the symptoms and effects of exposure to the chemical substance
Visual impairment is defined by the World Health Organization as exhibiting visual acuity of less than 6/12 in the better eye and by the American Academy of Ophthalmology.
When a person loses all or virtually all of their vision, it is said to be blindness. Vision is lost when someone is blind. Additionally, it could be used to describe a vision loss that cannot be treated with spectacles or contact lenses. You have very little vision if you are partially blind. You cannot see anything and you cannot see light if you are completely blind. When people refer to "blindness," they typically mean total blindness.
Learn more about blindness here brainly.com/question/14051134
#SPJ4.
O=H-H
is an acid,
a base,
Or
neither an
acid nor a
base.

Answers
The given structure is of formaldehyde an organic compound and it is acidic in nature.
Why is acidic formaldehyde?The formic acid is transformed into formaldehyde when hydrogen is added. Because of this, ambient oxygen can more quickly convert formaldehyde into formic acid. In addition to most polar organic solvents, formic acid is miscible with water. Although formaldehyde is a weak acid (pK greater than 13), there was no reliable method to estimate and correct the base bound by formaldehyde because the base bound by wool was always identified by comparing the base present at equilibrium in aliquots of solutions that were identical except for the presence of wool in one of them.Formaldehyde is a combustible, colorless gas that is noticeable for its strong aroma when it is at ambient temperature. Oxomethane, methylaldehyde, oxymethyline, and methanal are some of its other names.For more information on formaldehyde kindly visit to
https://brainly.com/question/29550668
#SPJ1
WIll give brainliest! : In the following Punnett square, what is the phenotypic percentages of the offspring? From dwarfism slideshow - length of legs.

Answers
Answer:
75% will have long legs and 25% will have short legs
Explanation:
Look at the word equation below. Which of the three products of this reaction is useful in cooking for making cakes rise?
sodium hydrogencarbonate → sodium carbonate + carbon dioxide + water
Answers
Three products of this reaction is useful in cooking for making cakes rise is sodium carbonate
Here given reaction is
sodium hydrogen carbonate → sodium carbonate + carbon dioxide + water
NaHCO₃ → Na₂CO₃ + CO₂ + H₂O
The baking powder decomposed during cooking and released carbon dioxide gas and this make the cake rise by putting bubbles of gas in it and baking powder is included in the recipe to make the cake light and fluffy and the baking powder makes the syrup and mixture puff up and turn into a honeycomb with lots of little bubbles in it and it happen because the baking powder break down to form carbon dioxide and carbon dioxide is a gas and it causes the bubble in the mixture
Know more about cooking
https://brainly.com/question/2924658
#SPJ1