Answers
Common use for a type of electromagnetic radiation is Thermal imaging uses infrared waves.
When magnetic field comes in contact with electric field than electromagnetic radiations are formed. The magnetic field and the electric field are perpendicular to each other. types of electromagnetic waves are : Radio waves, Microwaves, Infrared waves, Visible light , Ultraviolet waves, X- rays , gamma rays.
A tanning bed uses the ultraviolet waves
water purification uses the UV waves
Thermal imaging uses the infrared waves
weather radar use the Radio waves
Thus, Common use for a type of electromagnetic radiation is the Thermal imaging uses infrared waves.
To learn more about electromagnetic waves here
https://brainly.com/question/4284608
#SPJ1
Related Questions
Which characteristic of life best describes the process of photosynthesis?
Answers
Answer:
Using energy.
Explanation:
A runner exerting a running force of 325 N to the right is met with an air resistance force of 45 N to the left. What is the net
force of the runner? (1 point)
O 45 N
O 280 N
O 370 N
O 325 N
Answers
The net force of the runner, given the data from the question is 280 N
Data obtained from the questionFrom the question given above, the following data were obtained:
Exerting force = 325 NResistance force = 45 NNet force =?How to determine the net forceThe net force of the runner can be obtained as illustrated below:
Net force = Exerting force - resistance force
Net force = 325 - 45
Net force = 280 N
From the calculation made above, we can conclude that the runner's net force is 280 N
Learn more about net force:
https://brainly.com/question/13051854
#SPJ1
The net force of the runner would be 280 N.
Hope this helps! Also, if you need help with the other questions regarding this assignment, feel free to check out the study set I made on Quizlet. Thanks and good luck!
Quizlet - "Unit 6: Lesson 2 Newton's First Law QC"
h t t p s : / / q u i z l e t. c o m / 7 3 7 7 5 9 5 5 5 / u n i t - 6 - l e s s o n - 2 - n e w t o n s - f i r s t - l a w - f l a s h - c a r d s / ? n e w
Phthalonitrile (C₈H₄N₂) is produced by the ammoxidation of o-xylene (C₈H₁₀) according to the following reaction:
C₈H₁₀(l) + O₂(g) + NH₃(g) → C₈H₄N₂(s) + H₂O(l)
How many moles of water would be produced by the complete ammoxidation of 145.0 grams of o-xylene?
Answers
0.6066 moles of water are produced by complete ammoxidation of 145 g of o-xylene.
How are number of moles calculated?Number of moles are calculated by dividing the mass by the molar mass of the substance.In the reaction between o-xylene ,ammonia and oxygen pthalonitrile is produced.
Here, the number of o-xylene is calculated by the above mentioned formula which is 1.365 moles.Then, as per stoichiometry and balanced chemical equation ,
1.365 moles of o-xylene×8 moles of water/1 mole of xylene ×18 g water
=0.6066 moles
Thus,0.6066 moles of water are produced from 145 g of o-xylene.
Learn more about mole concept ,here:
https://brainly.com/question/22540912
#SPJ1
Can you classify solid and liquid please
Answers
Answer:
Solid : The intermolecular space is very less
Liquid : The intermolecular space is fairly more than in solids
Solid : It has a fixed shape
Liquid : It has a fixed volume but shape changes according to where it is placed
Solid : eg : Books
Liquid : eg : water
If my answer helped, kindly mark me as the brainliest!!
Thank You!!
Answer:
Liquids have the following characteristics:
No definite shape (takes the shape of its container).
Has definite volume.
Particles are free to move over each other, but are still attracted to each other
Explanation:
ye
g Oxidation of an aldehyde produces a ketone. primary alcohol. carboxylic acid. secondary alcohol. tertiary alcohol.
Answers
Explanation:
Oxidation of aldehyde yields carboxylic acid.
a ketone
This is incorrect
b primary alcohol
This is incorrect.
c carboxylic acid
This is the correct option
d. secondary alcohol
This is incorrect.
e. tertiary alcohol
This is incorrect
Oxidation of aldehyde yields carboxylic acid. An aldehyde gains oxygen and loses hydrogen during the oxidation process
A chemical reaction known as oxidation involves the loss of electrons or an increase in an element, atom, or compound's oxidation status. When a material reacts with oxygen, it frequently happens, but it can also involve other chemical substances or elements that serve as electron acceptors.
An aldehyde gains oxygen and loses hydrogen during the oxidation process, creating a carboxylic acid as a byproduct. Typically, oxidising substances like potassium dichromate or potassium permanganate are used to carry out this process in an acidic or alkaline media.
To know more about Oxidation, here:
https://brainly.com/question/13182308
#SPJ6
How many atoms are there in 4.00 moles of glucose, C6H12O6?
Answers
Answer:
4 moles of glucose = 4 x 6.022 x 10^23 = 2.4088 x 10^24 molecules of glucose.
There are 2.4088 x 10²⁴ atoms in 4 moles of glucose C₆H₁₂O₆
1 mole of any gas is defined as the number of atoms, molecules or ions present in the gas. 1 mole of any gas equals to 6.022 x 10²³ atoms , ions or molecules . 6.022 x 10²³ is known as avogadro's number.
We need a conversion factor that will converts from moles of glucose, C₆H₁₂O₆ to molecules of glucose.
That is, one mole of any substance contains 6.022 x 10²³ molecule of that substance.
4moles of glucose = 4 x 6.022 x 10²³ = 2.4088 x 10²⁴ molecules of glucose.
To know more about moles here
https://brainly.com/question/29162139
#SPJ2
which si unit would be most appropriate for describing the lengeth of the shoe?
Answers
Doeowknsnd cjxjznnzns sm
PLEASEEE HELPPP MEEEEE WITH THIS QUESTIONNN!!!!!
I REALLY NEED A GREAT RESPONSE AND ILL MARK YOU BRAINLIEST FOR YOUR EFFORT!!!!!!!
QUESTION: THINK CRITICALLY: Some conifers have female cones on the top half of the tree and male cones on the bottom half. Why would this arrangement of cones on a tree be important?
Answers
Answer:
The male cones appear on the trees in the spring time. The male cones are smaller than the female cones that we typically think of when we think of pine cones. These cones are softer and are only on the trees in the spring. After they release their pollen they die away and disappear.
Explanation:
Use the data to answer the questions that follow a. Determine the rate law
Answers
Answer:
To determine the rate law from a table, you must mathematically calculate how differences in molar concentrations of reactants affect the reaction rate to figure out the order of each reactant. Then, plug in values of the reaction rate and reactant concentrations to find the specific rate constant.
Explanation:
hope it is helpful for you
Watts are a measurement of power
Answers
Answer:
Watts are a measurement of power, describing the rate at which electricity is being used at a specific moment. For example, a 15-watt LED light bulb draws 15 watts of electricity at any moment when turned on. Watt-hours are a measurement of energy, describing the total amount of electricity used over time.
Please mark as brainliest.
Answer: True
Watts are a measurement of power, used in light bulbs and other electricity. It is also measured as candela (C) in SI Units. The answer to the question is true because of this measurement.
Hope this helps!
Please answer this!
What are the half-reactions for electrolytic cell with aluminum and gold
electrodes?
A. Al³+ (aq) + 3e → Al(s) and Au(s) → Au* (aq) + e
B. Al³+ (aq) + 3e → Al(s) and Aut(aq) + e¯ → Au(s)
► Au(s).
C. Al(s) → A1³+ (aq) + 3e and Au* (aq) + e →
D. Al(s) → Al³+ (aq) + 3e¯ and Au(s) → Au*(aq) + e

Answers
What happens to a solute?

Answers
Answer:
A
Explanation:
have a great day!!!!!!!!
The atomic number of an atom is the number of in the nucleus.
Answers
Taking into account the definition of atomic number, The atomic number of an atom is the number of protons in the nucleus.
Subatomic particlesAll atoms are made up of subatomic particles: protons and neutrons, which are part of their nucleus, and electrons, which revolve around them. Protons are positively charged, neutrons are neutrally charged, and electrons are negatively charged (electrons).
Definition of atomic numberThe atomic number of a chemical element is the total number of protons that each atom of that element has and is symbolized by the letter Z.
A neutral atom has the same number of protons in the nucleus and electrons in the orbitals, so the atomic number also indicates the number of electrons.
Learn more about atomic number:
brainly.com/question/5527493
#SPJ1
If you found a Carbon 13 atom, you would know that
Answers
To determine the absolute age of rocks and fossils, geologists use _____.
Answers
Answer:
The rates of decay of radioactive elements
Explanation:
The age of a rock in years is called its absolute age. Geologists find absolute ages by measuring the amount of certain radioactive elements in the rock. When rocks are formed, small amounts of radioactive elements usually get included.
Help, chemistry is so hard.

Answers
Oxidation in chemistry occurs when the atoms of an element lose electrons and the oxidation state of the element increases while reduction occurs when the atom of an element gains electrons and reduces the oxidation number.
The atom being reduced is the oxidizing agent while the atom being oxidized is the reducing agent.
Oxidation number is the hypothetical charge of an atom within a molecule. The oxidation number of an element can be calculated as follows;
V2O52x - 2(5) = 0
2x - 10 = 0
2x = 10
x = 5
Al2O32x - 6 = 0
2x = 6
x = 3
Learn more about oxidation and reduction at: https://brainly.com/question/13182308
#SPJ1
The bright-line spectra of four elements, G,J, L, and M, and a mixture of at
least two of these elements are given below.
Which elements are present in the mixture?
M
Mixture
750
750
G and J
G and L
M, J, and G
M, J, and L
700
700
650
650
Bright-Line Spectra
600
600
550 500
550
Wavelength (nm)
500
450
450
400
400
.
Answers
Based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
From the given bright-line spectra and the spectrum of the mixture, we can determine the elements present in the mixture by comparing the specific wavelengths observed. Examining the bright-line spectra, we can identify that G has a distinct wavelength at 650 nm, J at 600 nm, L at 550 nm, and M at 500 nm.
Looking at the spectrum of the mixture, we can observe two prominent wavelengths, 650 nm and 600 nm. These correspond to the wavelengths of G and J, respectively. Since the spectrum of the mixture does not exhibit the wavelengths specific to L (550 nm) or M (500 nm), we can conclude that only G and J are present in the mixture.
Therefore, based on the given bright-line spectra and the observed wavelengths in the mixture's spectrum, the elements G and J are the ones present in the mixture.
This analysis relies on the principle that each element has characteristic wavelengths at which they emit light. By comparing the observed wavelengths in the mixture's spectrum with those of the individual elements, we can determine the elements present in the mixture.
Know more about wavelengths here:
https://brainly.com/question/10750459
#SPJ8
what does the number 4 represent in 4nh3
Answers
the means there is 4 compounds of nh3
In \(\rm 4\;NH_3\), the 4 has been the coefficient of the compound and represents the number of molecules of ammonia.
\(\rm NH_3\) has been a compound of nitrogen and hydrogen. The compound has been a covalent compound, formed by the sharing of electrons in between nitrogen and hydrogen.
The compound has been consisted of 1 nitrogen atom and 3 hydrogen atoms. The hydrogen atoms bonding results in the completion of the octet of the nitrogen and provides stability to the compound.
While writing the chemical equation, the coefficient of the compound has been describing the number of molecules present in the reaction. In \(\rm 4\;NH_3\), the 4 has been the coefficient of the compound and represents the number of molecules of ammonia.
For more information about molecular formulas, refer to the link:
https://brainly.com/question/14425626
How many moles in 5 grams?
Answers
Answer:
The molar mass of atoms of an element is given by the standard relative atomic mass of the element multiplied by the molar mass constant
Explanation:
Why do you think plant cells need a cell wall as well as a cell membrane?
Answers
Answer:
to protect it from harm
Explanation:
The ratio of effusion ratios between He(g) and SF6(g) is
Answers
rate He = 6 x rate SF₆
Further explanationGraham's law: the rate of effusion of a gas is inversely proportional to the square root of its molar masses or
the effusion rates of two gases = the square root of the inverse of their molar masses:
\(\rm \dfrac{r_1}{r_2}=\sqrt{\dfrac{M_2}{M_1} }\)
or
\(\rm M_1\times r_1^2=M_2\times r_2^2\)
M He = 4 g/mol
M SF₆ = 146 g/mol
\(\tt \dfrac{r_{He}}{r_{SF_6}}=\sqrt{\dfrac{146}{4} }=6.04\approx 6\\\\r_{He}=6~r_{SF_6}\)
Can someone please explain to me how to do these questions ?

Answers
The common logarithm by exponentiating all sides with base 10 we obtain [H+] = 10pH. In a solution with a pH near 2, the pOH is 12, the hydrogen ion concentration is 10-2 M, and the hydroxide ion concentration is 10-2 M.
If a solution's H+ content is 10 8, what is its pH?The negative logarithm of the hydrogen ion concentration is used to calculate the pH of a solution. Change the hydrogen ion's value in the equation above. Hence, the pH of [10 - 8] 6.98 M HCl solution.
What pH is a solution with such a 10–12 M H+ concentration?A solution with such a concentration of hydroxyl ions of 1012 M has a pH of 2. As the pOH is indeed the numerical expression of the OH- ion concentration, such a solution does have a pOH of 12. As the sum of the pH & pOH must equal 14, the pH must be 2.
To know more about hydroxide visit:
https://brainly.com/question/4251554
#SPJ1
Which of the following affect the amount by which the freezing point of liquid is lowered by the addition of a solute? More than one answer may be correct.
A. Whether or not the compound is ionic.
B. How soluble the solute is in the solvent.
C. The volume of the solvent.
D. The value of the freezing point for the pure solvent.
E. The identity of the chemical species being dissolved.
Answers
The options that affect the amount that the freezing point is lowered by the addition of the solute include :
A. Whether or not the compound is ionic.B. How soluble the solute is in the solvent.How can solutes affect the freezing point of liquids ?The increasing of a solvent's boiling point as a result of the addition of a solute is known as boiling point elevation. Similar to freezing point depression, adding a solute lowers the freezing point of a solvent. In actuality, a solvent's freezing point drops as its boiling point rises.
Any solvent's freezing point will be lowered by the presence of a solute; this action is known as freezing-point depression. The fact that the solute is present in the liquid solution but not in the pure solid solvent is crucial to understanding this phenomenon.
This includes the compound being ionic or the solubility of the solvent.
Find out more on freezing point at https://brainly.com/question/40140
#SPJ1
Put the steps of the carbon cycle in order using Step 1 as your starting point.
Step 1: Bacteria, through nitrogen fixation and nitrification, convert nitrogen into a usable form.
The animal dies and decomposes, returning nitrogen back to the soil.
Once nitrogen is in usable form, it is taken up by plants and assimilated into proteins..
An animal eats a plant and the nitrogen becomes part of the animal’s proteins.
Answers
Answer:
Carbon moves from the atmosphere to plants. ...
Carbon moves from plants to animals. ...
Carbon moves from plants and animals to soils. ...
Carbon moves from living things to the atmosphere. ...
Carbon moves from fossil fuels to the atmosphere when fuels are burned. ...
Carbon moves from the atmosphere to the oceans.
Explanation:
Answer:
step 4 , 2 , 3
Explanation:
What is the correct answer? Please
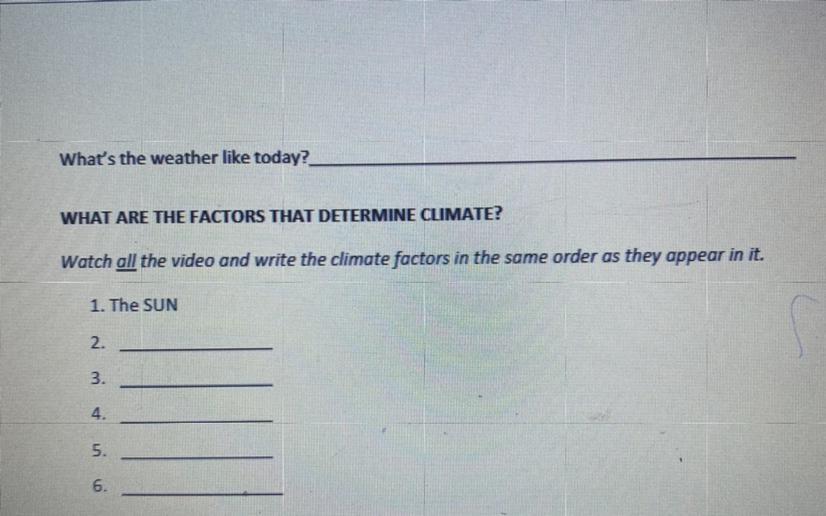
Answers
Answer:
Latitude
ocean currents
Wind
Elevation
Water
What is the mass in grams of 366mL of ethylene glycol
Answers
Answer:
\(m=406.26g\)
Explanation:
Hello,
In this case, since the density is defined as the degree of compactness of a substance and it mathematically defined as the ratio of the mass and volume:
\(\rho = \frac{m}{V}\)
By knowing that its density is 1.11 g/mL, the mass in 366 mL is:
\(m=\rho V=366mL*1.11\frac{g}{mL}\\ \\m=406.26g\)
Regards.
A typical engagement ring has 0.77 cm^3 of gold. What mass is present?
Answers
Answer:
Mass = 14.876 g
Explanation:
Given data:
Volume of gold = 0.77 cm³
Mass of gold = ?
Solution:
Density of gold from literature is 19.32 g/cm³
Formula:
d = m/v
d = density
m = mass
v = volume
by putting values,
19.32 g/cm³ = m/ 0.77 cm³
m = 19.32 g/cm³ × 0.77 cm³
m = 14.876 g
Pick a "for every" statement about copper and silver nitrate for this reaction.
A For every 1 mole of copper, the reaction uses 2 moles of silver nitrate.
B For every 1 mole of copper, the reaction makes 2 moles of silver nitrate.
C For every 1 mole of copper, the reaction uses 3 moles of silver nitrate.
D For every 1 mole of copper, the reaction uses 1 moles of silver nitrate.
Answers
From the equation of the reaction; for every 1 mole of copper, the reaction uses 2 moles of silver nitrate.
What is a reaction?A chemical reaction involves the transformation of one chemical specie into another. The reaction is not shown here hence the question is incomplete.
However, the reaction should be of the sort; Cu + 2AgNO3 ---> Cu(NO3)2 + Cu. Thus, for every 1 mole of copper, the reaction uses 2 moles of silver nitrate.
Learn more about chemical reaction: https://brainly.com/question/6876669
Glucose, C6H12O6, is used to prepare intravenous feeding solutions. What volume of 5.0 % W/V glucose solution can be prepared using 125 g of glucose? Show your working.
Please if the answer is correct, ill give brainliest
Answers
250 L of 5.0% w/v glucose solution can be prepared using 125 g of glucose.
We use the below formula to solve our problem,w/v = [ mass of solute (g) / volume of solution (mL) ] × 100
Substitute the values from our problem,5.0 % w/v = [ 125 g / volume of solution (mL) ] × 100
Rearranging the formula, we havevolume of solution (mL) = [ 125 g / 5.0 % w/v ] x 100
Substitute further for w/v,volume of solution (mL) = [ 125 g / (5.0 / 100) ] x 100
Simplify the expression,volume of solution (mL) = [ 125 g / 0.05 ] x 100
Hence, the volume of solution (mL) = 250,000 mL or 250 L1. Calculate the mass of a liquid with a density of 3.2 g/mL and a volume of 25 mL.
2. An irregular object with a mass of 18kg displaces 2.5 L of water when placed in a large overflow container. Calculate the density of the object.
3. A graduated cylinder has a mass of 80 g when empty. When 20 mL of water is added, the graduated cylinder has a mass of 100 g. If a stone is added to the graduated cylinder, the water level rises to 45 mL and the total mass is now 156 g. What is the density of the stone?
Answers
Explanation:
incidido 4itkjrnej que me ha gustado un vídeo que 7, que es el caso caso de que la gente que te guste. no se