which of the following molecules can form hydrogen bonds? select one: a. ch4 b. nah c. nh3 d. bh3 e. hi
Answers
Answer:
nh3
Explanation:
:) let me know if its right!
Related Questions
I NEED HELP ASAP 7 and 8 pls will mark brainlist

Answers
Answer:
6. the box with more particles in it will be more dense than the same box with fewer particles, so basically a
LITHIPODIUS NULLA
Which members of the population are the most adapted? Least adapted?
Answers
Answer:According to the Darwin’s theory “ the survival of the fittest” , any organism would survive through the process of natural selection by adapting itself to the changing environment in order to become the fittest among all of its native species.
Thus, in order to survive even after some major environmental changes, an organism is required to adapt to the new conditions.
Hence, option 2 is the correct answer which talks about adaptation as per the changing environmental condition
Explanation:
Which of the following best describes the impact of glaciers upon the surface of the earth?
a.
Ice buried continents under a few hundred feet of ice.
b.
Glaciers scraped across the surface and altered landscapes and the course of rivers.
c.
The passage of glaciers created new ecosystems.
d.
All of the above.
Answers
Answer:
New Answer is : B - Glaciers scraped across the surface and altered landscapes and the course of rivers.
Explanation:
Edge 2020
The impact of glaciers on the surface is that glaciers scraped across the surface and altered landscapes and the course of rivers.
What is a glacier?A glacier refers to a heap of ice that has stayed there for a large number of years. The earth is filled with many glaciers and about 90% of fresh water are trapped in glaciers.
The impact of glaciers on the surface is that glaciers scraped across the surface and altered landscapes and the course of rivers.
Learn more about glaciers: https://brainly.com/question/14309099
If you dissolve 0. 3 moles of hbr in 1 l of water, what do you expect to be present in the beaker after the compounds have had time to react?
a. H2Br, OH^-, and H2O
b. H3O^+, Br^-, and H2O
c. BrOH and H3O^+
d. HBr, H3O^+, Br^-, and H2O
Answers
After the chemicals had a chance to react, the components HBr, H₃O⁺, Br⁻, & H₂O will anticipate being present in the beaker. Hence, the correct choice is option D.
Generally, water and the diatomic molecule hydrogen bromide (HBr) combines together to form the caustic acid also known as hydrobromic acid. The pH of HBr is usually 0.21. Generally a very acidic environment with corrosive liquid (HBr) are produced when an extremely poisonous and miscible in water gets compressed gases bromine is dissolved into the water.
Generally, HBr is soluble in water because of its ability to form interparticle hydrogen bonds with water. In HBr, usually a dipole forms because of the disparity in electron density between H and Br. Hence, option D is correct.
Learn more about chemicals from the link given below.
https://brainly.com/question/13145357
#SPJ4
How did Dalton explain the formation of compounds?
A) Atoms divide into smaller parts.
B) Atoms chemically combine.
C) Mixtures of atoms are formed.
D) Copies of atoms are made
Answers
Dalton explained the formation of compounds by Atoms chemically combine. Option B is correct.
According to the Dalton's atomic theory, atoms are indivisible and they will combine in fixed ratios to form a compounds. He proposed that elements will consist of tiny, indivisible particles termed as atoms that will combine in simple, whole-number ratios to form a compounds.
He believed that atoms of different elements combine in the fixed ratios to form compounds, and that the ratio of their masses determines the ratio of their combining atoms.
Thus, Dalton explained that the formation of the compounds can occurs through a chemical combination of atoms, rather than through the division or copying of atoms.
Hence, B. is the correct option.
To know more about Dalton's atomic theory here
https://brainly.com/question/11855975
#SPJ4
average atomic mass of zync
Answers
Answer:
The average atomic mass of zinc is 65.38 u.
Explanation:
Hope this helps.
Give brainliest please!
Realice una historieta que resuma su comprensión acerca de la teoría atómica y los diferentes modelos atomicos que se
han propuesto a lo largo de la historia.
Answers
Respuesta:
Los modelo atómicos han permitido representar el modo de funcionamiento de los átomos. A lo largo de la historia han surgido un numero de modelos atómicos diferentes incluyendo los modelos de Bohr, Thomson, Rutherford, Sommerfeld, Dalton y Schrödinger.
Explicación:
El modelo atómico propuesto por John Dalton (1808) demostró que las sustancias químicas reaccionan en proporciones fijas y cómo mediante su combinación se producen elementos diferentes. Dalton fue el primero en postular la existencia de elementos indivisibles llamados átomos. A continuación, Thomson (1904) desarrolló un modelo en el cual el átomo estaba compuesto por protones con carga positiva y electrones con carga negativa los cuales se incrustaban uniformemente dentro de este átomo, asemejándose a las pasas de uva de un budín. En 1911, Ernest Rutherford desarrolló un nuevo modelo donde la masa principal del átomo tenía carga positiva y se localizaban en el núcleo, mientras que los electrones con carga negativa se posicionaban en la región externa del átomo. Subsecuentemente, Niels Bohr (1913) represento el funcionamiento del átomo de hidrógeno mediante un protón inmóvil en el núcleo atómico y un electrón girando a su alrededor. El modelo atómico de Sommerfeld permitió generalizar el diagrama de Bohr a otros tipos de átomos mas allá del Hidrógeno, incluyendo diferentes niveles energéticos para cada átomo particular. El modelo de Schrödinger (1926) permitió corregir aquellas discordancias surgidas del modelo atómico de Bohr. Schrödinger incluyó diferentes niveles y subniveles de energía a los electrones e incorporó órbitas elípticas a su movimiento, con lo cual permitiendo predecir los efectos relativos de los campos magnético y eléctrico sobre el movimiento de los electrones.
5. A scientist mixed two samples together: a white
solid that boils at about 800°C and a colorless gas
that boils at about 70°C. He analyzed the results and
found two ending substances. One of the ending
substances boils at 245°C. This ending substance is
made up of the repeating group of atoms shown
above. Which of the diagrams to the left shows the
repeating groups of atoms that make up the
samples the scientist mixed together?
Answers
The starting material can be shown by the image in image B
What is a chemical reaction?A chemical reaction is a process that involves the conversion of one or more substances (called reactants) into new substances (called products) through the breaking and forming of chemical bonds. In a chemical reaction, the original chemical properties of the reactants are changed, resulting in the formation of one or more new substances with different properties.
Chemical reactions can involve the combination of two or more substances to form a single product, the decomposition of a single substance into two or more products, or the exchange of atoms or groups of atoms between reactants to form new products.
Learn more about chemical reaction:https://brainly.com/question/1329342
#SPJ1

If 6. 02x10^1c9 he atoms are found in 2. 0 mol of gas, what is the he mole fraction in ppm?.
Answers
To find the mole fraction of He in the gas, we first need to calculate the total number of moles of gas in the sample. Since 6.02x10^23 atoms make up one mole of gas, we can calculate the number of moles in the sample by dividing the number of atoms by Avogadro's number:
6.02x10^23 atoms / 1 mole = 2.0 moles of gas
Next, we need to calculate the mole fraction of He in the gas. This is the ratio of the number of moles of He to the total number of moles of gas in the sample:
Mole fraction of He = moles of He / total moles of gas
Mole fraction of He = 6.02x10^19 / 2.0
Mole fraction of He = 3.01x10^19
Finally, to express the mole fraction in parts per million (ppm), we need to multiply by 10^6:
Mole fraction of He (in ppm) = 3.01x10^19 x 10^6
Mole fraction of He (in ppm) = 3.01x10^25 ppm
Therefore, the mole fraction of He in the gas sample is 3.01x10^25 ppm.
Hi! I'd be happy to help you with your question. To find the helium (He) mole fraction in parts per million (ppm), follow these steps:
1. Calculate the total number of moles of gas: We are given that there are 2.0 moles of gas in total.
2. Calculate the moles of He atoms: We are given 6.02 x 10^19 He atoms. To convert atoms to moles, divide by Avogadro's number (6.022 x 10^23 atoms/mol):
(6.02 x 10^19 atoms) / (6.022 x 10^23 atoms/mol) ≈ 0.0001 mol He.
3. Calculate the mole fraction of He: Divide the moles of He by the total moles of gas:
(0.0001 mol He) / (2.0 mol gas) = 0.00005.
4. Convert the mole fraction to ppm: Multiply the mole fraction by 1,000,000:
0.00005 x 1,000,000 = 50 ppm.
So, the helium mole fraction in the given gas mixture is approximately 50 ppm.
To know more about mole fraction visit
https://brainly.com/question/8076655
#SPJ11
true or false:all elements with high ionization energy also have high electron affinity
Answers
Hello!!!
Answer: Therefore elements with high ionization energies have more positive electron affinity whereas alkali metals having the lowest ionization energy do not tend to add electrons.
Explanation: So energy is to be supplied for the addition of electrons and shows positive electron affinity.
Hope This Helps You Out! So... The Answer Your Looking For Is "True"
The statement is true as all elements with high ionization energy have more electrons and hence have high electron affinity.
What is an element?
It is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
Elements can be classified as metals and non-metals. Metals are shiny and conduct electricity and are all solids at room temperature except mercury. Non-metals do not conduct electricity and are mostly gases at room temperature except carbon and sulfur.
The number of protons in the nucleus is the defining property of an element and is related to the atomic number.All atoms with same atomic number are atoms of same element.
Learn more about element,here:
https://brainly.com/question/14347616
#SPJ2
The air in the balloon i heated up by leaving it in a warm place. Give two effect that thi ha on the air particle
Answers
If the balloon is closed, then yes, both volume and pressure will increase when the gas inside is heated.
What is pressure?
Pressure is the force applied perpendicular to the surface of an object per unit area over which that force is distributed.
Various units are used to express pressure. Some of these are units of force divided by units of area. For example, the SI unit of pressure, Pascal (Pa), is 1 Newton per square meter (N/m2). Similarly, pounds force per square inch (psi, symbol lbf/in2) is the traditional unit of pressure in imperial and US systems. Pressure can also be expressed as standard atmospheric pressure. Atmospheric pressure (atm) is equal to this pressure and torr is defined as 1/760 of this. Manometric units such as centimeters of water, millimeters of mercury, and inches of mercury are used to express pressure as the height of a particular liquid column within a manometer.
If the balloon is closed, then yes, both volume and pressure will increase when the gas inside is heated.
To know more about Pressure, visit:
https://brainly.com/question/28012687
#SPJ4
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
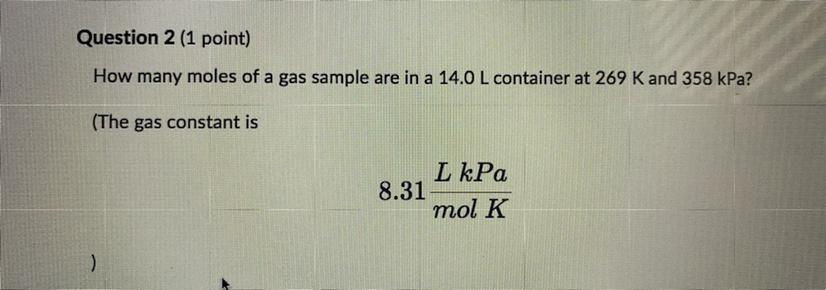
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
if two molecules of glucose (c6h12o6) are joined via condensation synthesis, the resulting molecule would have a molecular formula of .
Answers
The condensation reaction between two molecules of glucose would result in the formation of maltose (C12H22O11) and the release of water.
Carbohydrates are organic compounds that are used to store energy. An example of a carbohydrate is glucose, which is a single sugar or a monosaccharide. The process of forming larger molecules from the combination of simple sugars is called the condensation process. A water molecule is released in the process of combining the molecules together through a glycosidic bond.
The condensation reaction for two molecules of glucose (C6H12O6) results in the formation of maltose, a disaccharide, and the release of water. The molecular formula of maltose is C12H22O11. It can be observed that the condensation reaction shown below is balanced.
Glucose (C6H12O6) + Glucose (C6H12O6) → Maltose (C12H22O11) +H2O
To learn more regarding the condensation reaction of sugars, please refer to https://brainly.com/question/24950735.
#SPJ4
Which of the following sets of quantum numbers represents the last electron added to an arsenic atom (As)? n=4, l=2,m=0n=3, l=2, m=0n=3, l=1, m=0n=4, l=1, m=2n=4, l=1, m=0
Answers
The set of quantum numbers that represents the last electron added to an arsenic atom (As) is n=4, l=1, m=2.
An electron is a negatively charged particle that orbits the atomic nucleus in the atom's outermost shell. The electron contains practically no mass but has a charge of -1.
Quantum numbers help to explain and comprehend the structure of electrons. An electron is described by four quantum numbers, which give it a unique identification in a given atom's electronic configuration.
The sets of quantum numbers are: n, l, m, and s. Where n represents the principle quantum number, l is the angular momentum quantum number, m represents the magnetic quantum number, and s is the spin quantum number.
In the case of the last electron added to an arsenic atom (As), the set of quantum numbers that represents it is n=4, l=1, m=2. This is because the last electron added will fill in the outermost shell of the atom.
Therefore, the principle quantum number (n) is equal to 4 because the valence shell is in the fourth shell. l, which is the angular momentum quantum number, is equal to 1 because it is the valence shell's subshell.
Finally, m, the magnetic quantum number, is equal to 2 because of the orientation of the orbital in which the electron is present. As a result, the answer is n=4, l=1, m=2.
To know more about quantum numbers, refer here:
https://brainly.com/question/28943816#
#SPJ11
which of the following is an anthropogenic source of sulfur dioxide? a barbecue grill that runs on natural gas a jogger out of breath in a marathon volcanic eruptions coal-burning power plants
Answers
Coal-burning power plants is an anthropogenic source of sulfur dioxide
Anthropogenic sources refer to human activities that contribute to the release of certain substances or pollutants into the environment. In this case, coal-burning power plants are known to be a significant anthropogenic source of sulfur dioxide (SO2) emissions. When coal is burned as a fuel in power plants, it releases sulfur dioxide into the atmosphere as a byproduct of combustion. This is a major contributor to air pollution and can have detrimental effects on human health and the environment. The other options listed, such as a barbecue grill running on natural gas, a jogger out of breath in a marathon, and volcanic eruptions, are not typically associated with significant anthropogenic sulfur dioxide emissions.
Know more about sulfur dioxide here:
https://brainly.com/question/9720549
#SPJ11
1. The characteristics of an acid are that it has a _____________ taste, reacts with _____________, and turns litmus paper into ______________.
2. When you add TOO MUCH solute to a solution, the solution becomes ____________________.
3. A mixture contains a ___________________ and a ___________________.
4. You test a liquid and its pH is 7. This liquid is _____________________.
5. Lemon juice, apple juice, and vinegar all have pH measurements below seven (7), making them a (n) __________.
6. Ammonia, blood, and drain cleaner all have pH measurements above seven (7), making them a (n) ________________.
7. ___________________ is another word for eating away at material.
8. __________ can be an acid or base because it is a good conductor of electricity.
9. __________ is a positive ion and __________ is a negative ion.
10. Two or more substances mixed but not chemically combined are a _____________.
Answers
Answer:
Explanation:
1. The characteristics of an acid are that it has a sour taste, reacts with metals, and turns blue litmus paper into red.
2. When you add TOO MUCH solute to a solution, the solution becomes saturated at some point. It will not dissolve anymore and will remain solid instead.
3. A mixture contains an acid and a base.
4. You test a liquid, and its pH is 7. This liquid is Neutral.
5. Lemon juice, apple juice, and Vinegar all have pH measurements below seven (7), making them acidic.
6. Ammonia, blood, and drain cleaner all have pH measurements above seven (7), making them basic.
7. Erosion is another word for eating away at material.
8. Vinegar can be an acid or base because it is a good conductor of electricity.
9. Cation is a positive ion and Anion is a negative ion.
10. Two or more substances mixed but not chemically combines are a Mixture.
The sodium-potassium exchange pump transports potassium and sodium ions in which direction(s)?
Sodium ions are transported out of the cell. Potassium ions are transported into the cell.
channel-mediated diffusion
a chemical gradient going out of the cell and an electrical gradient going into the cell
Answers
The sodium-potassium exchange pump transports sodium ions out of the cell and potassium ions into the cell. So, the correct answer is sodium ions out of the cell and potassium ions into the cell.
The sodium-potassium exchange pump is a transport protein found in the plasma membrane of most cells, which uses energy in the form of ATP to transport potassium and sodium ions across the membrane. Specifically, for every ATP molecule that is hydrolyzed, the pump transports three sodium ions out of the cell and two potassium ions into the cell.
This pump is important for maintaining the proper ion concentration gradient across the plasma membrane, which is essential for various cellular processes such as nerve impulse transmission and muscle contraction. The movement of sodium ions out of the cell and potassium ions into the cell occurs against their concentration gradients, which means that the pump moves these ions from areas of lower concentration to areas of higher concentration.
Therefore, the sodium-potassium exchange pump transports sodium ions out of the cell and potassium ions into the cell. This process is different from channel-mediated diffusion, which involves the movement of ions down their concentration gradients through ion channels. The movement of ions through ion channels can be influenced by both chemical and electrical gradients, depending on the charge and concentration of the ions involved.
For more such questions on potassium
https://brainly.com/question/24527005
#SPJ11
An ice skater moves back and forth on an ice rink. She speeds up on the straight portion of the rink. She slows down near each end of the rink and then turns around. Which claim supported by evidence?
Answers
The options are;
A) The skater experiences acceleration both while skating in a straight line and while making her turn.
B) Because the skater does not change her direction or speed, she does not experience acceleration.
C) The skater only experiences acceleration when she is turning.
D) The skater only experiences acceleration while skating in a straight line.
Answer:
A: The skater experiences acceleration both while skating in a straight line and while making her turn
Explanation:
We are told that She speeds up on the straight portion of the rink. This means that she experienced an acceleration since the speed was not constant but gradually increasing.
Also, we are told that She slows down near each end of the rink and then turns around. This means that she reduced speed before turning and it means there was also an acceleration as well when making the turn.
Thus, she experience acceleration both in the straight line and when making turn.
Option A is correct
Find solutions for your homework
science
chemistry
chemistry questions and answers
given the following set of data, determine the enthalpy of formation of the nacl(s) from elemental na(s) and cl2(g) given the following information. lattice energy for solid nacl(s):-742kj/mol enthalpy of sublimation for solid na(s)->na(g):108kj/mol first ionization energy for sodium:496kj/mol cl-cl bond dissociation energy:75.3kj/mol electron affinity for
This problem has been solved!
You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
See Answer
Question: Given The Following Set Of Data, Determine The Enthalpy Of Formation Of The NaCl(S) From Elemental Na(S) And Cl2(G) Given The Following Information. Lattice Energy For Solid NaCl(S):-742kJ/Mol Enthalpy Of Sublimation For Solid Na(S)->Na(G):108kJ/Mol First Ionization Energy For Sodium:496kJ/Mol Cl-Cl Bond Dissociation Energy:75.3kJ/Mol Electron Affinity For
Given the following set of data, determine the Enthalpy of formation of the NaCl(s) from elemental Na(s) and Cl2(g) given the following information.
Lattice Energy for solid NaCl(s):-742kJ/mol
Enthalpy of sublimation for solid Na(s)->Na(g):108kJ/mol
First ionization energy for sodium:496kJ/mol
Cl-Cl bond dissociation energy:75.3kJ/mol
Electron affinity for chlorine:-349kJ/mol
If possible please type your answer and explanation.
Answers
The enthalpy of formation of NaCl(s) from elemental Na(s) and Cl₂(g) is -411.65 kJ/mol.
The enthalpy of formation for NaCl(s) can be calculated using the given data as follows;
Na(s) → Na(g)
ΔH1 = +108 kJ/mol
1/2Cl₂(g) → Cl(g)
ΔH2 = +121 kJ/mol
Na(g) → Na+(g) + e-
ΔH3 = +496 kJ/mol
Cl(g) + e- → Cl-(g)
ΔH4 = -349 kJ/mol
Na+(g) + Cl-(g) → NaCl(s)
ΔH5 = -742 kJ/mol
The enthalpy of formation of NaCl(s) from elemental Na(s) and Cl₂(g) can be calculated using Hess’s law.
Therefore;
ΔH5 = ΔH1 + ΔH2 + ΔH3 + ΔH4
Now, substituting the given data into the above equation we get;
-742 kJ/mol = +108 kJ/mol + 1/2(+121 kJ/mol) + (+496 kJ/mol) + (-349 kJ/mol) + ΔH4
Therefore;
ΔH4 = -75.3 kJ/mol
Thus, the enthalpy of formation of NaCl(s) from elemental Na(s) and Cl₂(g) is given as;
ΔH5 = ΔH1 + ΔH2 + ΔH3 + ΔH4= 108 kJ/mol + 1/2(121 kJ/mol) + 496 kJ/mol - 349 kJ/mol - 75.3 kJ/mol
= -411.65 kJ/mol
Therefore, the enthalpy of formation of NaCl(s) from elemental Na(s) and Cl₂(g) is -411.65 kJ/mol.
To know more about Hess’s law, visit:
https://brainly.com/question/31508978
#SPJ11
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
Anything helps thank you

Answers
3 x 2 because double the C3N
Vast quantities of natural gas are located beneath Earth’s surface. Natural gas is less costly to extractfrom the ground than crude oil. Natural gas offers some advantages compared to other energy sources. Despite the large amount of natural gas, why is it classifies as a nonrenewable, rather than a renewable,resource?
Answers
Natural gas is classified as a nonrenewable resource rather than a renewable resource due to the following reasons:
1. Limited Availability: Natural gas is formed over millions of years through the decomposition of organic matter buried deep within the Earth's crust. The formation process takes a significant amount of time, and the rate at which natural gas is replenished is much slower compared to its consumption. This limited rate of formation makes it impractical to consider natural gas as a renewable resource.
2. Finite Reserves: The reserves of natural gas are not infinite. While there are vast quantities of natural gas located beneath the Earth's surface, these reserves are still finite and will eventually be depleted if extraction continues at the current rate. Once the natural gas reserves are exhausted, they cannot be replenished within a human timescale.
3. Environmental Impact: Although natural gas is considered a cleaner-burning fossil fuel compared to coal and oil, its extraction and combustion still have environmental impacts. The extraction of natural gas can lead to habitat destruction, water pollution, and the release of greenhouse gases such as methane, which contributes to climate change.
Based on these factors, natural gas is categorized as a nonrenewable resource because its availability is limited, its reserves are finite, and its extraction and use have environmental consequences.
Learn more about gas here:
https://brainly.com/question/14812509
#SPJ11
In a chemical equation, the number written in front of the chemical formula is the ____
Answers
which of the following molecules can form hydrogen bonds with others like it?
Answers
Answer:
nitrogen, oxygen and fluorine
2.) the lym.an series is brighter than the balmer series because this series of transitions ends up in the most common state for hydrogen, the ground state. why then was the balmer series discovered first?
Answers
The balmer series for hydrogen was discovered first because it lies in the visible domain of the spectrum, although the lym.an series is brighter because it exhibits a series of transitions in its more common state.
What is the balmer series?It is the set of lines derived from the emission of the hydrogen atom when an electron transits from a level n ≥ 3 to n = 2. n represents the principal quantum number referring to the energy level of the electron.
What is the lyman or lym series?It is the set of lines produced by the emission of the hydrogen atom when an electron transits from n ≥ 2 to n = 1.
There is another series called Paschen series, which corresponds to a level less than or equal to 3
Learn more about the balmer series at https://brainly.com/question/14915323
#SPJ4

how can you avoid the formation of the side product in this experiment? group of answer choicesby drying the aqueous layer.by using pure sodium iodide.by using pure silver nitrate.by keeping the reaction at a low temperature and avoiding overheating the product during distillation.by venting the gases out of the separatory funnel.
Answers
This is because side reactions and unwanted byproducts are often favored at higher temperatures.
To avoid the formation of a side product in this experiment, the best approach would be to keep the reaction at a low temperature and avoid overheating the product during distillation. This is because side reactions and unwanted byproducts are often favored at higher temperatures. By maintaining a low temperature, the reaction can be controlled to favor the desired product and minimize the formation of side products.
Drying the aqueous layer, using pure sodium iodide, using pure silver nitrate, and venting the gases out of the separatory funnel are not specifically related to preventing the formation of side products in this context. They may be relevant for other aspects of the experiment, but they would not directly address the formation of side products.
Learn more about reactions here
https://brainly.com/question/16737295
#SPJ11
25.As a solution becomes more acidic, the pH of the solution...Select one:a. increases.b. decreases.c. remains unchanged.d. quickly increases and then gradually decreases.
Answers
Answer:
\(B\text{ : decreases}\)Explanation:
Here, we want to know what happens to a solution that becomes more acidic
A lesser ph (1-7) indicates acidity with the acidity being higher as the number becomes smaller
What this means is that a solution with a pH of 3 is more acidic than a solution with a pH of 5
Thus, when the acidity increases, it is expected that the pH of the solution decreases (it becomes smaller in number)
an aqueous solution of gold nitrate, au(no3)3 undergoes electrolysis in order to plate on a necklace, requiring 0.50 hours using 4.50 amperes of current. what mass of gold in grams is plated on the necklace? faraday's const
Answers
5.52 grams mass of gold will be plated on the necklace.
What is mass?
Faraday's constant is 96,485 Coulombs per mole.
To calculate the mass of gold plated on the necklace, we need to know the amount of charge that has passed through the electrolytic cell during the electrolysis process. We can calculate the charge using the equation:
charge = current x time
charge = 4.50 A x 0.50 h x 3600 s/h = 8100 C
Next, we need to calculate the moles of electrons involved in the electrolysis process. Each mole of \(Au(NO_{3})_{3}\) requires 3 moles of electrons to reduce \(Au_{3}^{+}\) to metallic gold. So, the moles of electrons is:
moles of electrons = charge / Faraday's constant / 3
moles of electrons = 8100 C / (96,485 C/mol) / 3 = 0.0280 mol
Finally, we can calculate the mass of gold using the molar mass of gold, which is 196.97 g/mol:
mass of gold = moles of electrons x molar mass of gold
mass of gold = 0.0280 mol x 196.97 g/mol = 5.52 g
Therefore, 5.52 grams of gold will be plated on the necklace.
To know more about gold, visit:
https://brainly.com/question/16585110
#SPJ1
Complete question is: an aqueous solution of gold nitrate, \(Au(NO_{3})_{3}\)undergoes electrolysis in order to plate on a necklace, requiring 0.50 hours using 4.50 amperes of current. 5.52 grams mass of gold is plated on the necklace.
Explain why the following are made of thermosetting plastics.
(a) Saucepan handles
(b) Electric plugs/switches/plug boards
Answers
Answer:
a: they're used in saucepan handles 'cause they don't soften when heated and also 'cause they cant be bent easily.
b: thermosetting plastics are bad electricity conductors. they don't get moulded and are also hard and strong.
What is the purpose of adding sodium sulfate to the organic layer after extraction?.