Answers
Hey,The Answer is Milk, Go for it!!
Related Questions
Which scientist discovered the neutron and have us the current basic model of the atom?
Answers
Answer:
james Chadwick discovered it
HELP ME GUYS I NEED HELP RN

Answers
Answer:
Robot 1 : 150 J
Robot 2: 40 J
Explanation:
30 N x 5m = 150 J
20 N x 2m = 40 J
Please mark as brainliest
Have a great day, be safe and healthy
Thank u
XD
Covalent compounds Need you guys

Answers
a. The names for the chemical compounds in the task given above are as follows:
CO₂: Carbon dioxideP5N7: Pentaphosphorus heptanitride.CO: Carbon monoxideP5Br3: Pentaphosphorus tribromideSF6: Sulfur hexafluorideN2O8: Nitrooxyperoxy nitrateSCl4: Sulfur tetrachlorideSi2Br9: Disilicon nonabromideMg3P2: Magnesium phosphideBaO: Barium oxideCa3N2: Calcium nitrideLiI: Lithium iodideb. The formula for the compound given above are:
Chlorine dioxide: ClO₂Dinitrogen trioxide: N₂O₃Phosphorus pentoxide: O₁₀P₄Dihydrogen monoxide: H₂OHexaboron monosilicide: B6SiChlorine dioxide: ClO₂Hydrogen monoiodide: HIHexaboron hexasilicide: B6Si6.What is a compound?It is a substance formed between two two or more different elements which are chemical compound together and whose properties are entirely different from the properties of the contribution elements.
In conclusion, we can now confirm from the explanation given above that a compound could be ionic or molecular.
Read more on compounds:
https://brainly.com/question/26487468
#SPJ1
(Will give brainlest if right)Which substance can be broken down chemically ? A) Ammonia B)aluminium C)Argon
Answers
Answer:
i believe its b but i could be wrong
True or false? The subscripts in a chemical formula do not change for a given compound.
Answers
True. The subscripts in a chemical formula represent the relative number of atoms of each element in a compound. They indicate the ratio of atoms present and remain constant for a given compound.
Changing the subscripts would alter the composition and stoichiometry of the compound.
forces between the negatively charged electron and the positively charged nucleus, allowing the electron to be completely removed from the atom. It is typically measured in units of electron volts (eV) or kilojoules per mole (kJ/mol). Ionization energy is influenced by factors such as the atomic structure, electron shielding, and the effective nuclear charge experienced by the outermost electrons. The ionization energy generally increases as you move across a period in the periodic table due to increased nuclear charge and decreased atomic radius. It also decreases as you move down a group due to increased electron shielding and atomic size. Ionization energy plays a crucial role in understanding chemical reactions, electron configurations, and the reactivity of elements.
Learn more about chemical here:
https://brainly.com/question/29240183
#SPJ11
definition of these 20 words
density
hollow
solid
conserved
conduction
convention
current
emit
expand
rediation
vigorously
porous
evaporation
isopropanol
condence
temperature
density
kinetic energy
thermal energy
condensation
mass
→chemistry←⊕
Answers
Given what we know, we can give the definition of these 20 words.
Density: magnitude that shows the correlation between mass and volume of a solution.
Hollow: having an empty space or concavity.
Solid: state of matter in which its particles are together and organized with great cohesion among themselves.
Conserve: to keep or protect something from some kind of damage or change.
Conduction: transmission of a charge through a body.
Convention: an established technique, form, or practice.
Current: a flow of electrically charged particles that travels through space.
Emit: release or discharge something such as a type of gas, liquid, light or sound.
Expand: increase the volume in a given space by some change in temperature.
Radiation: transmission of energy from a source through space.
Vigorously: done energetically or actively.
Porous: body that allows some fluid to pass through it.
Evaporation: it is a physical process in which it goes from the liquid state to the gaseous state. It can occur at any temperature
Isopropanol: colorless, flammable alcohol that can be mixed with water.
Condense: state of matter in which particles are packed closely together.
Temperature: thermal level of a body or the atmosphere.
Kinetic energy: energy possessed by a body by its motion.
Thermal energy: internal energy enclosed in a thermodynamic system in equilibrium that is proportional to its absolute temperature.
Condensation: is the change of matter from a gaseous state to a liquid state.
Mass: magnitude with which we measure the amount of matter that expresses a body.
To learn more about chemistry visit: https://brainly.com/question/17066001?referrer=searchResults
#SPJ1
Which of the following is an example of chemical weathering?
O A. Water freezes in cracks in a rock, breaking the rock apart.
O B. Weak acid breaks down minerals by reacting with them.
O C. Plant roots grow and split rock into pieces.
O D. An animal kicks a rock down a hill where it hits another rock and shatters.
Answers
Answer:O B. Weak acid breaks down minerals by reacting with them.
Explanation:
Chemical weathering is the process whereby rain water which sometimes can be acidic ( As rain falls down it reacts with CO2 in the atmosphere and form acid rain), This reacts with the minerals contained in rocks, dissolves and degrades them further to form entire new minerals.
An example of a rock that is greatly affected by overexposure of acid rain is limestone containing calcite which easily degrades by acid rain.
Other processes whereby Chemical weathering can occur are through reaction with water and oxygen.
The first law the of thermodynamic also known as the "Law of Conservation of Mass" states that
A. heat changes occur during chemical and physical changes.
B. there are two types of energy, kinetic and potential
C. In any chemical or physical change, energy cannot be created or destroyed, only transformed in form.
D. energy is the capacity to do work or to supply heat
Answers
In any chemical or physical change, energy cannot be created or destroyed, only transformed in form.
option C.
What is the first law of thermodynamics?The first law of thermodynamics is known as the law of Conservation of Energy.
This law states that energy can neither be created nor destroyed but can be converted from one form to another.
So the first law of thermodynamics is not known as the "Law of Conservation of Mass", but rather as the "Law of Conservation of Energy".
The statement that best corresponds to the first law of thermodynamics is option C: "In any chemical or physical change, energy cannot be created or destroyed, only transformed in form."
Learn more about first law of thermodynamics here: https://brainly.com/question/26035962
#SPJ1
Everything else being equal, a gradual increase in atmospheric CO2 would most likely bring
about: (1) no change in global climate (2) a decrease in evaporation from the earth's oceans
(3) a marked decrease in plant growth
(4) an increase in surface temperature
Answers
Everything else being equal, a gradual increase in atmospheric CO2 would most likely bring about (4) an increase in surface temperature.
CO2 is a greenhouse gas, which means that it absorbs infrared radiation and contributes to the warming of the Earth's atmosphere. As the concentration of CO2 in the atmosphere increases, it traps more heat and leads to an increase in surface temperature. This effect is known as the greenhouse effect.
The increase in temperature can have many consequences, including changes in precipitation patterns, rising sea levels, and more frequent and severe weather events. However, it is important to note that other factors, such as changes in solar radiation and volcanic activity, can also affect the Earth's climate.
Learn more about temperature here:
https://brainly.com/question/11464844
#SPJ11
Someone pls help me I will mark you as brain
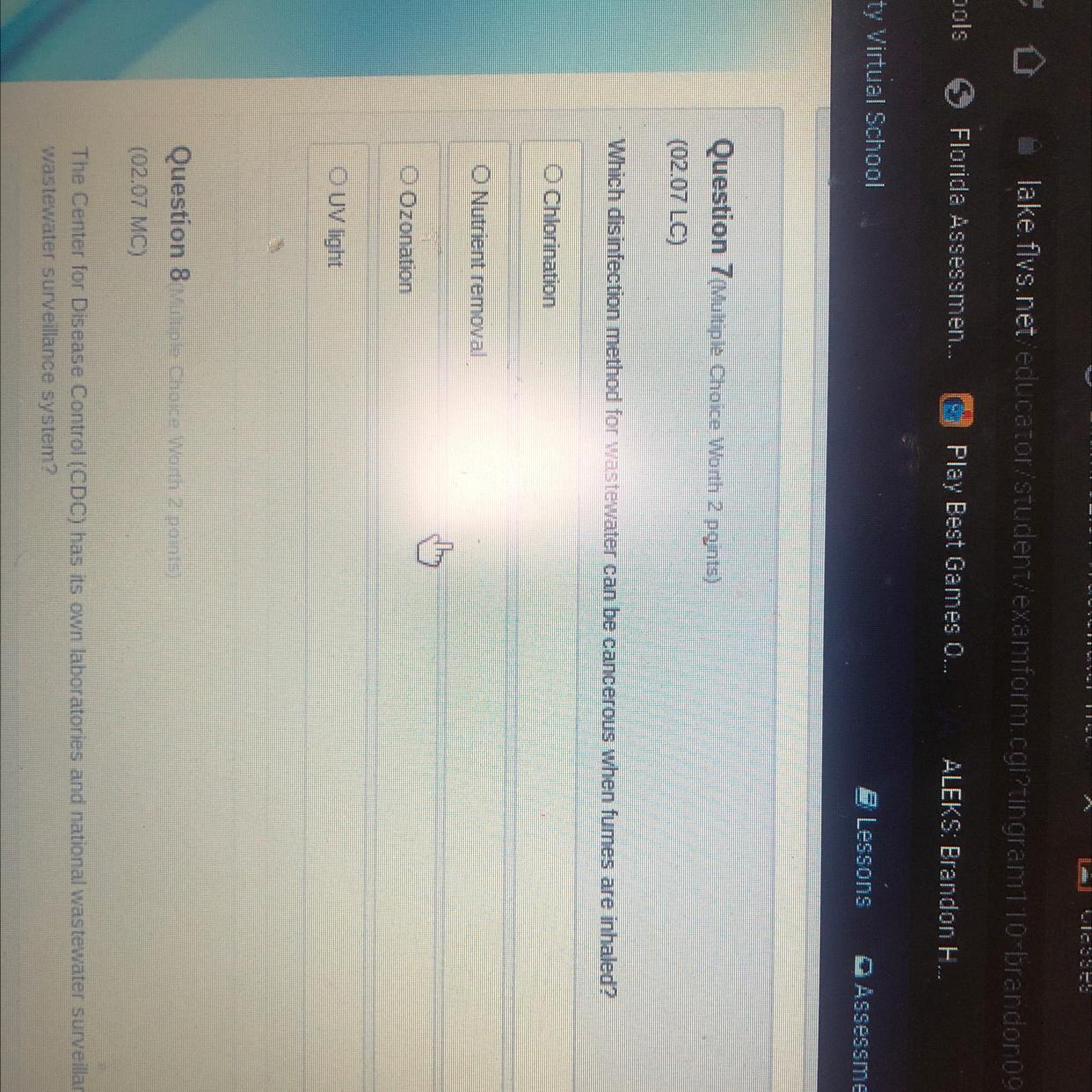
Answers
Answer:
Chlorination
Explanation:
"Exposure to high volumes of chlorine gas fumes can cause serious health problems, including death."
https://water.mecc.edu/courses/ENV211/lesson14_print.htm
:)
What type of solution do you create if you put 50g of NH4Cl in 100g of water at 70 degrees C
Answers
Answer:
hi
Explanation:
1. How many miles are in 1,000,000 centimeters?

Answers
Answer:
6 miles.
Explanation:
There is a lot of decimal points after it. But you just need to convert everything. But if you round it to the nearest whole, it would be 6 miles.
Answer:
Ex1,000,000 Centimeters =
6.2137119 Miles
planation:
Balance the following redox reaction in acidic solution. ClO3– + Cl– → Cl2 + ClO2
What is the sum of the coefficients in the balanced redox reaction?
Answers
The sum of the coefficients in the balanced redox reaction is 17.
In the given redox reaction, we need to balance the equation in acidic solution. The first step is to balance the atoms in the reaction by adding coefficients in front of each compound. We start by balancing the atoms that appear in multiple compounds. By assigning appropriate coefficients, we balance the number of chlorine (Cl) atoms on both sides of the equation.
Next, we balance the number of oxygen (O) atoms by adding water molecules (H₂O) to the equation. We also balance the charge by adding hydrogen ions (H⁺) to the reaction. Finally, we check the overall charge and atom balance to ensure the equation is balanced. After balancing, the coefficients of the balanced equation are: ClO₃⁻ + 6Cl⁻ → 5Cl₂ + ClO₂. The sum of the coefficients is 17, which is the answer to the question.
Learn more about Redox reactions
brainly.com/question/28300253
#SPJ11
Answer and explain please

Answers
Answer:
I can only see b
Explanation:
Answer:
Carbon- 11 has 6 protons in its nucleus and boron- 11 has 5 protons in its nucleus.
Explanation:
note: Carbon has an atomic number of 6
and Boron has an atomic number of 5.
Can someone help me with my chemistry problem?

Answers
Answer:
D. 108g of water
Explanation:
16g CH4 produces 2(18)g of H20
1g CH4 produces \(\frac{36}{16}\)g of H2O
48g CH4 produces \(\frac{36}{16}\)×48
108g of H20
Answer:
D. 108g of water
Explanation:
how do i convert 0.5L to mL
Answers
Answer: 500
Explanation: there's 0.001L in every mL. Divide the mL
by 1000 or multiply the liter by 1000.
which one of the following would form a precipitate with poâ³⻠ions in aqueous solution?A. K+B. NHâ+C. Ca2+D. None of these would form a precipitate
Answers
We have established that the reaction among Pb(NO3)2 aquatic and MgSO4 aqueous in answer option (A) is the only scenario which is something a deposit is likely to develop.
Why did MgSO4 get used?Copper sulfate is a medication used to treat and prevent seizures and low blood magnesium levels in eclamptic women. Additionally, it is used to treat barium poisoning, acute asthma exacerbations, torsades de pointes, and constipation.
What medication is MgSO4?The most popular medication for treating eclampsia and preventing it in people with severe which was before is magnesium sulfate (MgSO4). Generally, it is administered intravenously and otherwise intramuscularly.
To know more about MgSO4 visit:
https://brainly.com/question/8691104
#SPJ4
Many industrial companies and car manufacturers design solutions to reduce pollution. Even so, chemicals still enter the atmosphere. Some of these pollutants combine with water in the air to form acid rain.
Which property of acids would cause acid rain to damage buildings over time?
A. Acids taste sour.
B. Acids react with metals.
C. Acids react with limestone.
D. Acids react with indicators.
I THINK THE ANSWER IS (C)
Part 2 of the question
What might be done to prevent acid rain damage to objects from metal and carbonate, such as limestone? Identify a metal object that could be damaged by acid rain. Then, describe what could be done to prevent acid rain damage to it. Do the same thing for an object that contains carbonate
Answers
The property of acids which would cause acid rain to damage buildings over time is:
Acids react with limestone.
The correct answer choice is option c.
This simply means that acids has this property which corrodes the surface they come in contact with especially stones.
One of those things which can be done to prevent acid rain damage to objects from metal and carbonate, such as limestone is coating the surface of the metal.However, this can be done by coating the surface of metals, or carbonate by coating it with other metals. By so doing, this can prevent acid rain damage to it.
That being said, by default, concentrated acids are highly corrosive.
Coating surfaces of metals can prevent acid rain damage to it
What is an acid?This refers to a substance which when dissolved in water, it produces hydrogen ion as the only positive ion in the solution.
So therefore, the property of acids which would cause acid rain to damage buildings over time is acids react with limestone.
Learn more about acid:
https://brainly.com/question/8676275
#SPJ1
How many joules are required to heat a frozen can of juice (360 grams) from -5 °C (the temperature of an
overcooled refrigerator) to 110 °C (the highest practical temperature within a microwave oven)?
Answers
It would require approximately 19,008 joules of energy to heat a frozen can of juice (360 grams) from -5 °C to 110 °C.
To calculate the energy required to heat the frozen can of juice from -5 °C to 110 °C
We need to use the specific heat capacity formula:
Q = mcΔT
Where
Q is the amount of energy required (in joules) m is the mass of the can of juice (in kilograms) c is the specific heat capacity of the juice (in joules per kilogram per degree Celsius)ΔT is the change in temperature (in degrees Celsius)First, we need to convert the mass of the can of juice from grams to kilograms:
m = 360 g = 0.360 kg
Next, we need to find the specific heat capacity of the juice. The specific heat capacity varies depending on the type of juice, but for the purposes of this calculation, we can assume a value of around 4200 J/kg°C, which is the specific heat capacity of water.
c = 4200 J/kg°C
Finally, we can calculate the energy required to heat the can of juice:
Q = mcΔT
Q = (0.360 kg)(4200 J/kg°C)(110°C - (-5°C))
Q = (0.360 kg)(4200 J/kg°C)(115°C)
Q = 19,008 J
Therefore, it would require approximately 19,008 joules of energy to heat a frozen can of juice (360 grams) from -5 °C to 110 °C.
Learn more about heat capacity here : brainly.com/question/21406849
#SPJ1
If 20 ml of water is added to a solution of ethanoic acid with a ph of 3, how will the solution change? a. the concentration of h will increase. b. the amount of h will increase. c. the amount of h will decrease. d. the concentration of h will decrease.
Answers
The addition of 20 ml of water to a solution of ethanoic acid with a pH of 3 will cause the concentration of H+ ions to decrease, leading to a decrease in the amount of H+ ions present in the solution.
The pH scale is a measure of the concentration of H+ ions in a solution. A pH of 3 indicates a relatively high concentration of H+ ions. When water is added to the solution, it will dilute the ethanoic acid and increase the total volume of the solution. However, since water is neutral and does not contribute H+ ions, the concentration of H+ ions will decrease as the overall volume increases. This results in a decrease in the amount of H+ ions present in the solution. Therefore, the correct answer is d. The concentration of H+ ions will decrease.
Learn more about pH scale here:
brainly.com/question/1431406
#SPJ11
An electric motor works by converting electrical energy to mechanical energy in order to create motion.
For example,Force is generated within the motor through the interaction between a magnetic field and winding alternating (AC) or direct (DC) current.
This works because…
Answers
An electric motor works by converting electrical energy to mechanical energy in order to create motion.
For example,Force is generated within the motor through the interaction between a magnetic field and winding alternating (AC) or direct (DC) current.
This works because…
Answer : When an electric current flows through the wire in the electromagnet, a magnetic field is produced in the coil.
\(\:\mathsf{Hope\:this\:helps\:you!}\)
Answer:
when an electric current flows through the wire in the electromagnet , a magnetic field is produced in the coil .
Explanation:
Hope it's helpful for you .........Which of the following statements are true of the maintenance of blood pressure (BP)?
Answers
C. If blood pressure decreases, filtration in the kidneys decreases.
D. If more blood returns to the heart, the ventricles contract more forcefully to pump it out, and this raises blood pressure.
What is the maintenance of blood pressure?The maintenance of blood pressure is defined as the high use of sodium in your diet and the low use of potassium in your diet can cause hypertension. It is also important to use those foods which are free from fats. It is also important that we should use fruits, vegetables, and grains in excessive amounts for the maintenance of blood pressure and for a healthy diet.
High blood pressure 190/110 mm Hg can cause the main vital organs of the body like the brain, heart, and kidney too. There are three factors that contribute to blood pressure which are resistance, blood viscosity, and blood vessel diameter.
So we can conclude that blood pressure is the pressure of circulating blood against the walls of the blood vessels.
Learn more about Blood Pressure here: https://brainly.com/question/25149738
#SPJ1
Which of the following statements are true of the maintenance
of blood pressure (BP)? (Read carefully and select all of the correct statements.)
A. Norepinephrine stimulates vasoconstriction in skin, viscera, and skeletal muscles, all of which lower BP.
B. Aldosterone stimulates the reabsorption of Na+ ions by the kidneys, which lowers BP.
C. If BP decreases, filtration in the kidneys decreases.
D. If more blood returns to the heart, the ventricles contract more forcefully to pump it out, and this raises BP.
E. If BP decreases, the kidneys secrete the enzyme renin, which stimulates the secretion of epinephrine to prevent a further decrease.
F. The hormone ANP increases the excretion of K+ ions by the kidneys, which helps conserve water and raise BP.
G. The hormone ADH helps the kidneys conserve water and helps prevent a decrease in BP.
H. The elasticity of the large arteries helps decrease diastolic BP.
Magnesium chloride crystals can be used by reaction which insuluble base with an acid?
Answers
Magnesium chloride crystals can be formed by reacting an insoluble base with an acid. The reaction can be represented by the following equation:
\(Mg(OH)2 + 2HCl → MgCl2 + 2H2O\)In this reaction, magnesium hydroxide (Mg(OH)₂) is the insoluble base and hydrochloric acid (HCl) is the acid. When these two substances react, they produce magnesium chloride (MgCl₂) and water (H₂O).
The magnesium chloride formed in this reaction is in the form of crystals. The crystals form because the reaction produces an excess of ions, which then attract to each other and form a lattice-like structure. This is known as crystallization. The magnesium chloride crystals are colorless and have a salty taste.
Learn more about Magnesium chloride at: https://brainly.com/question/28193925
#SPJ11
how many electrons occupy a filled 6s sublevel
Answers
Answer:
2 electrons
Explanation:
every s sublevel can only be occupied by 2 electrons so a filled 6s sublevel would have 2 electrons
Please balance the equation, putting the correct coefficient in each box. Do not leave any boxes blank! Enter "1" for formulas having a presumed coefficient of 1. F2 + P2 --> PF3
Answers
Answer:
3F2+P2_>2PF3now f is 6 on both sides and your p is also 2 on both sides
With what compound will NH3 experience only dispersion intermolecular forces?
A. CH4
B. LiCl
C. CH3Br
D. HOF
E. CH3OH
Answers
LiCl (lithium chloride) is an ionic compound and would not be expected to interact strongly with NH3 through intermolecular forces, The correct answer is A. CH4.
NH3 (ammonia) can experience several intermolecular forces, including dispersion forces, dipole-dipole forces, and hydrogen bonding. However, the strength of these forces depends on the nature of the other molecules present in the system.
Of the compounds given, only CH4 (methane) is nonpolar, meaning it only experiences dispersion forces with other nonpolar molecules. NH3 can also experience dipole-dipole forces with polar molecules, such as CH3Br (methyl bromide) and CH3OH (methanol), and hydrogen bonding with compounds that have a hydrogen atom bonded to a highly electronegative atom, such as HOF (hypofluorous acid) and water.
LiCl (lithium chloride) is an ionic compound and would not be expected to interact strongly with NH3 through intermolecular forces. Therefore, option B is not the correct answer.
Learn more about dipole-dipole forces here https://brainly.com/question/14195217
#SPJ4
I need help it’s due in a couple minutes !

Answers
Answer:
chemical composition
can someone help me with this?

Answers
Answer:
I see "photosynthesis" and "cellular respiration". I wonder if photosynthesis has something to do with the sun.
Explanation:
What concentration of ClO3â results when 967 mL of 0.367 M AgClO3 is mixed with 667 mL of 0.643 M Mn(ClO3)2?
Answers
Answer:
0.742M is the concentration of ClO₃⁻
Explanation:
The AgClO₃ contains 1 mole of ClO₃⁻ per mole of compound, but Mn(ClO₃)₂ contains 2 moles of ClO₃⁻ per mole of compound.
The moles of AgClO₃ are:
0.967L * (0.367moles / L) = 0.355 moles AgClO₃ = 0.355 moles of ClO₃⁻
And of Mn(ClO₃)₂:
0.667L * (0.643moles / L) = 0.429 moles Mn(ClO₃) * 2 = 0.858 moles of ClO₃⁻
That means total moles of ClO₃⁻ are:
0.355 moles + 0.858 moles = 1.213 moles ClO₃⁻ that are in 0.967L + 0.667L = 1.634L.
The concentration is:
1.213 moles ClO₃⁻ / 1.634L =
0.742M is the concentration of ClO₃⁻what is the function of the cell membrane?
Answers
Answer:
Its the thin lining around the cell the moniters what gets in and out of the cell.
Explanation:
