Answers
The pH of the methylamine solution is 10.11. It is calculated using the expression for pH of the solution.
Methylamine is classified as an example of weak base with K=3.7×10−4. These base partially ionizes in aqueous medium producing hydroxide ion and its conjugate acid, methylammonium that is a weak acid. some of the examples of weak base includes ammonia and dimethylamine.
pH is defined as the acidity denoting "potential of hydrogen" is a scale used to specify the acidity or basicity of an aqueous solution. According to the pH scale acidic solutions are measured to have lower pH values than basic or alkaline solutions. The pH of the solution containing methylamine and methylammonium chloride is given in the terms of,
pH = pKa + log[CH3NH2] / [CH3NH+3]
=−log1.0×10−14 / 3.7×10−4 + log 0.22M / 0.63M
=10.11
To learn more about pH
https://brainly.com/question/26424076
#SPJ4
Related Questions
How can you observe a plant giving off oxygen during photosynthesis
Answers
A plant giving off oxygen during photosynthesis comes from water .
The plants takes up the carbon dioxide and the water and in the presence of sunlight releases the oxygen as the by product in the process of the photosynthesis. in the process of the photosynthesis , the chlorophyll excited by the sunlight. in the presence of this the water undergoes the photolytic oxidation. this means that water is breaking down in to the hydrogen ion , electrons and the free oxygen. there are various experiments that shows that the source of oxygen is the water.
Thus, A plant giving off oxygen during photosynthesis comes from water .
To learn more about photosynthesis here
https://brainly.com/question/1388366
#SPJ1
plz help me with this question

Answers
Cars rust because when metal comes into contact with water or some sort of water form, it will make the surface wet. If the metal is wet for too long, it will start to soak up the water, therefore making it rusty. The salt will also rust a car. It does this because the mix of carbon dioxide and oxygen in the salt will eat away at the car, making it rust faster. Hope this helps!
Complete the table. Then explain how wavelength and frequency are mathematically related.
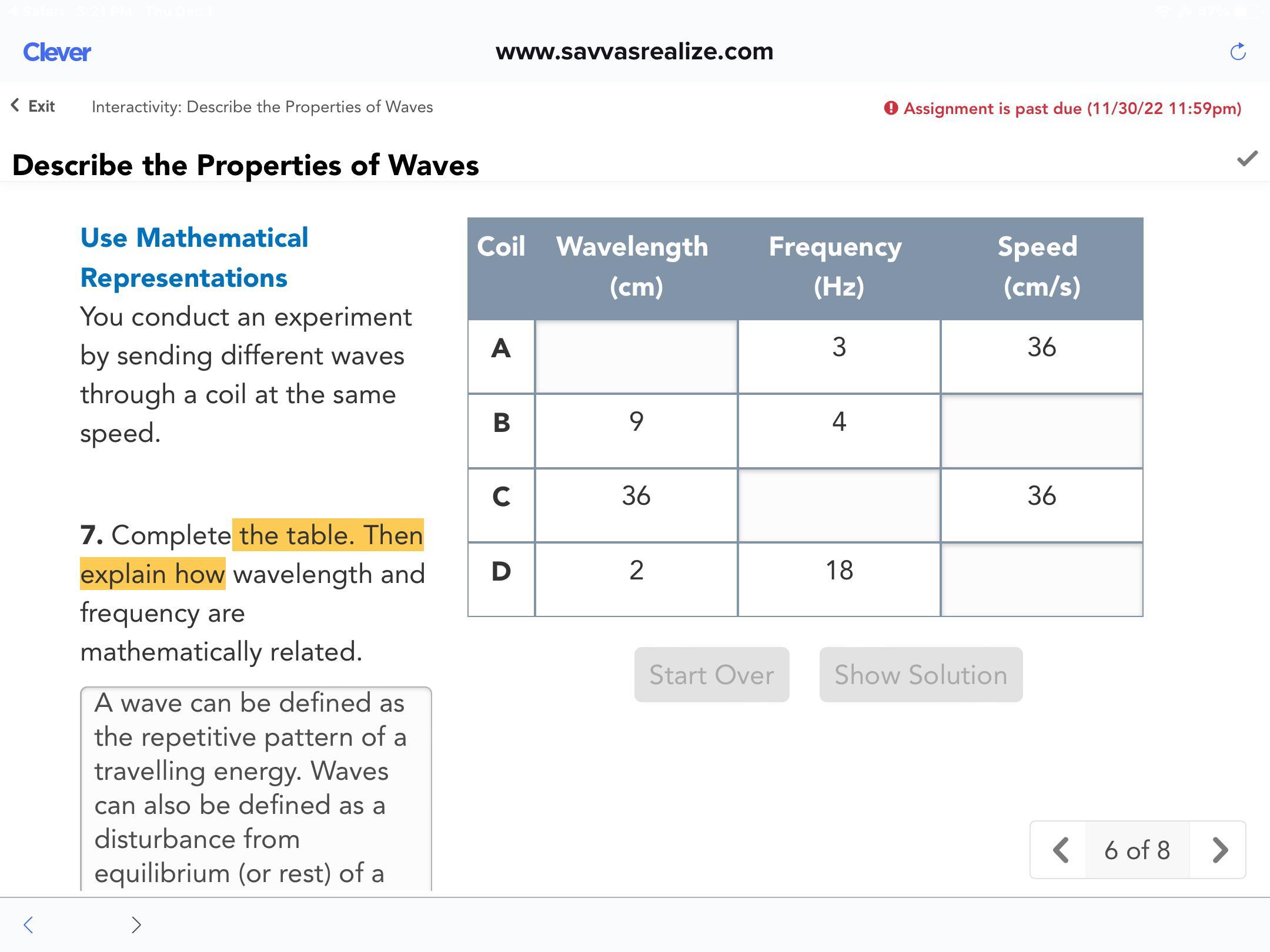
Answers
The wavelength is same.
What is wavelength?
Wavelength can be defined as the distance between two successive by throughs of a wave.
The question wants you to determine the energy that is the incoming photon by the must have in order to the accept the electron that melted it tob the jump to 2 to 1.
A good starting point here will be to calculate the energy of to the photon emitted when the electron falls from 1 to 2
by applying the Rydberg equation.
1/π = R
λ
si the wavelength of the emittted photon R
is the Rydberg constant, equal to 1.907.
This means that you haveλ=
4.10
So, you know that when an electron falls from the
to
a photon of wavelength by the
410 nm
is emitted. This implies by that in order to the for the electron by jump to the 2 to 1 .
it must absorb a photon of the same wavelength.
To know more about wavelentgh click-
https://brainly.com/question/10750459
#SPJ1
What is Na2Co3? How look like that's?
Answers
Sodium carbonate, often referred to as Na2CO3, is a chemical compound composed of atoms of sodium (Na), carbon (C) and oxygen (O).
It is also sometimes called washing soda or soda ash. At room temperature, sodium carbonate is a white, crystalline solid that is very soluble in water. According to the chemical formula of the sodium carbonate molecule, Na2CO3, each molecule consists of two sodium atoms (Na), one carbon atom (C) and three oxygen atoms (O). The atomic configuration in sodium carbonate is shown in the given diagram.
A trigonal planar arrangement is formed when the central carbon atom is bonded to three oxygen atoms. The structure of sodium carbonate is completed by two sodium atoms joined to oxygen atoms.
Learn more about Sodium carbonate, here:
https://brainly.com/question/31422792
#SPJ1

To _____ means to draw a conclusion based on something you observe
A. Guess
B. Control
C. Model
D. Infer
Answers
Answer: D
Explanation: Infer
The tomato is dropped. What is the velocity, v
, of the tomato when it hits the ground? Assume 86.0 %
of the work done in Part A is transferred to kinetic energy, E
, by the time the tomato hits the ground.
Express your answer with the appropriate units.
Answers
To determine the tomato's velocity when it hits the ground, we need more information. Specifically, we need the height from which the tomato was dropped and the tomato mass.
Without these details, it is impossible to calculate velocity accurately. The velocity of an object when it hits the ground depends on factors such as the height of the fall, the mass of the object, and any forces acting on it during the fall (such as air resistance).
If you can provide the necessary information, I can help you calculate the velocity of the tomato when it hits the ground.
1 mole of sulfur atoms has how much mass
Answers
Answer:
One atom of sulfur has a mass of 32.07 AMU; one mole of S atoms has a mass of 32.07 g.
Explanation:
Therefore, the answer should be 32.07 g
What is the frequency of a photon that has 3.82 x 10^-20 J of energy?
Answers
Answer:
5.73498*10^13
Explanation:
............

When 0.695 g of an unknown gas are held in an otherwise-empty 293-mL container, the pressure is 807.4 mmHg at 22°C. What is the molecular mass of the gas?
____g/mol
Answers
The molecular mass of the gas is 54 g/mol.
The Ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behaviour of many gases under many conditions, although it has several limitations. The ideal gas equation can be written as
PV = nRT
where,
P = Pressure
V = Volume
T = Temperature
n = number of moles
In this equation, number of moles can be replaced by mass divided by molecular mass.
Given,
Mass = 0.695g
Volume = 293ml = 0.293L
Pressure = 807.4mmHg = 807.4/760 atm
Temperature = 22⁰C = 22 +273 = 295K
PV = nRT
(807.4 × 0.293) ÷ 760 = (0.695 × 8.314 × 295) ÷ M
M = (0.695 × 0.0821× 295 ×760) ÷ (807.4 × 0.293)
M= 54 g/ mol
Learn more about Ideal gas Law, here:
https://brainly.com/question/28257995
#SPJ1
K2CR2O7 +H2SO4+FeSO4
Answers
Answer:
Refer to the attachment
hope it helps

Which reason best explains why metals are malleable?
A. because they have delocalized electrons
B. because they have localized electrons
C. because they have ionic bonds
D. because they have rigid bonds
Answers
Answer:
A
Explanation:
because they have delocalized electrons
Metals are malleable because they have delocalized electrons which are capable of forming ionic bonds.
What is an ionic bond?
Ionic bond or electrovalent bond is a type of bond which is formed between two elements when there is an exchange of electrons which takes place between the atoms resulting in the formation of ions.
When the atom looses an electron it develops a positive charge and forms an ion called the cation while the other atom gains the electron and develops a negative charge and forms an ion called the anion.
As the two atoms are oppositely charged they attract each other which results in the formation of a bond called the ionic bond.Compounds having ionic bonds are malleable and have high melting points.They are also brittle in nature.
Learn more about ionic bond,here:
https://brainly.com/question/11527546
#SPJ6
draw the lewis structure for a, brf5b. carbonate
Answers
a. T o draw the lewis structure of this molecule, we have to know the valence electrons of each of the element that make part of it. Bromine has 7 valence electrons and so does fluorine.
They are bonded by covalent bonds, then, the lewis structure is:
In this structure, the dots represent the valence electrons and the line the bonds, there are 5 fluorine atoms and 1 bromine atom.
b. Carbonate has the following formula CO₃²-. Carbon has 4 valence electrons and oxygen has 6, nevertheless, in this structure, 2 of the oxygens gained 1 electron each, which makes the structure to have a negative charge, the oxygen that has not an extra electron has a double bond with the carbon. The lewis structure is the following:
Remember to draw the negative charges to the oxygens that gained an electron.


Is sodium chloride an ionic compound?
Answers
sodium chloride is an ionic compound due to huge electronegative difference between the atom of the compound.
In other contexts, sodium chloride is known as salt. Both inland and coastal waters are affected. There is also rock salt available. In seawater, salt chloride levels range from 1% to 5%. It is a crystalline, white substance. When it is in an aqueous state, it is referred to as a saline solution. It is water soluble and contains sodium cation and chloride anion. One to one is the ratio of sodium to chloride ions. It is frequently referred to as table salt and is primarily used in the food industry for flavoring and preservation. The pH of sodium chloride is 7.
To know more about Sodium Chloride visit : brainly.com/question/14516846
#SPJ4
When heated, calcium carbonate, CaCO3(s) , decomposes to calcium oxide, CaO(s) , and carbon dioxide, CO2 . Using relevant data from your book's appendices, calculate the heat evolved or consumed when 15.0 g of calcium carbonate are decomposed. answer: kJ
Answers
As per the standard data, the heat evolved during one mole of calcium carbonate decomposes is 177.8 KJ. Thus 15 g or 0.15 moles of calcium carbonate when decomposed will produce 26.67 KJ of heat.
What is reaction enthalpy?Reaction enthalpy of a substance is the heat evolved or absorbed during a reaction. Reaction enthalpy is negative for an exothermic reaction and positive for an endothermic reaction.
Molar mass of calcium carbonate = 100 g.
no.of moles in 15 g = 15 /100 = 0.15 moles.
One mole or 100 g of calcium carbonate decompose to evolve 177.8 KJ according to the scientific record.
Thus, heat evolved by the decomposition of 0.15 moles is 0.15 × 177.8 KJ = 26.67 KJ.
Hence, the heat evolved during the decomposition of 15 g of calcium carbonate is 26.67 KJ.
To find more on reaction enthalpy, refer here:
https://brainly.com/question/1657608
#SPJ1
Uranium is an element with three naturally occurring isotopes: 238U, 235U, and 234U. This means that 238U, which has a mass number of 238 has _______more than 235 u which has a mass number of 235.
Answers
Answer:
The correct answer is - neutrons.
Explanation:
Uranium has various isotopes found naturally that are three 238U, 235U, and 234U. Uranium has an atomic number of 92 which means there are 92 protons and 92 electrons in the atomic structure.
Isotopes have the same number of protons but a different number of neutrons that can vary from 141 to 146. U-238 has 146 neutrons in the nucleus, whereas 235 U has 143 neutrons.
The U- 238 has more neutrons than U- 235. Atomic mass is the sum total of the nucleons or protons and neutrons.
What are Isotopes?
They are the different variants of the same molecules which have the same number of protons but the different number of neutrons.
Atomic mass is the sum total of the nucleons or protons and neutrons. The atomic number of Uranium is 92, the rest of the mass comes from neutrons.
Therefore, the U- 238 has more neutrons than U- 235.
Learn more about Isotopes:
https://brainly.com/question/9099776
The wavelength of yellow light is about 550nm. How many cm is it?
Answers
Answer:
\(5.5 \times {10}^{ - 5} \: cm\)
Explanation:
We know, that:
\(1nm \: = \: 1 \times {10}^{ - 7} \: cm \)
Now, we can make a proportion according to this:
1nm - 1×10^-7 cm
550 nm - x cm
\(x = \frac{550 \times 1 \times {10}^{ - 7} }{1} = 550 \times {10}^{ - 7} = 5.5 \times {10}^{ - 5} \: cm \: = 0.000055 \: cm\)
Which atomic models in task 1 are not supported by Thomson’s experimental evidence? For each of these models, explain the experimental results that the model would predict.
Answers
The atomic models in task 1 that are not supported by Thomson’s experimental evidence are Dalton's atomic model and Rutherford model.
Why are they not supported by Thomson’s experimental evidence?Dalton's atomic model proposed that atoms were indivisible and indestructible, and that atoms of different elements had different weights and properties. But Thomson's experiment, which found that atoms are divisible and can be split into smaller particles called electrons, disproved this model.
Also, Rutherford's atomic model proposed that atoms were made up of a dense central nucleus of protons and neutrons, surrounded by a cloud of electrons. But Thomson's experiment, which found that electrons are spread evenly throughout the atom and not concentrated in a central nucleus, disproved this model.
Learn more about atomic models from
https://brainly.com/question/20349334
#SPJ1
Complete and balance the following redox reaction in basic solution. Be sure to include the proper phases for all species within the reaction.
MnO4- (aq) + N2O3 (aq) -----------> Mn2+ (aq) + NO3- (aq)
Answers
3 MnO\(_4\)⁻ + 2N\(_2\)O\(_3\) + 2H\(_2\)O → 3Mn²⁺ + 2 NO\(_3\)⁻ + 6 OH⁻ is the balanced redox reaction in the basic condition.
Any chemical process in which a participating chemical species' oxidation number changes is known as an oxidation-reduction reaction, often known as a redox reaction. The phrase refers to a broad range of processes. Numerous oxidation-reduction processes are as frequent and well-known as fire, metal corrosion and disintegration, fruit browning, respiration, and photosynthesis—basic life processes.
3 MnO\(_4\)⁻ + 2 N\(_2\)O\(_3\) + 6 H⁺ + 6 OH⁻ → 3Mn²⁺ + 2 NO\(_3\)⁻ + 4 H\(_2\)O + 6 OH⁻
3 MnO\(_4\)⁻+ 2 N\(_2\)O\(_3\) + 6 H\(_2\)O → 3 Mn²⁺ + 2 NO\(_3\)⁻ + 4 H2O + 6OH⁻
3 MnO\(_4\)⁻ + 2N\(_2\)O\(_3\) + 2H\(_2\)O → 3Mn²⁺ + 2 NO\(_3\)⁻ + 6 OH⁻
To know more about redox reaction, here:
https://brainly.com/question/13293425
#SPJ1
In a chemical equation, the symbol that takes the place of the words reacts with is an) Example HCI + KOH-KCI + H2O
O equal sign
O coefficient
O plus sign
O arrow
Answers
Answer: c plus sign
Explanation:
The symbol that is used in the reaction is an equal sign, it is put between reactant and product. The correct option is A, an equal sign.
What is a chemical equation?The representation of a reaction in the equation form is known as a chemical equation.
In the given reaction, the hydrochloric acid and potassium hydroxide undergoes a reaction and produce potassium chloride and water.
The reactants and the products are always divided with an arrow or equal sign.
HCI + KOH = KCI + H2O
Thus, the correct option is A, an equal sign.
Learn more about chemical equation
https://brainly.com/question/12047033
Do you use sig figs in percent errors??

Answers
Answer:
I think you use sig figs in percent error.
show that the units of kinetic energy and of gravitational potential energy are the same.
Answers
Explanation:
We have to first obtain the unit of each of the individual energies.
Kinetic Energy
E = 1/2 mv²
The units are;
mass, m = Kg
Velocity, v = m⋅s−1
This means that;
E = Kg * (m⋅s−1)²
E = kg m² / s²
Gravitational Potential Energy
Pe = mgh
The units are;
mass, m = Kg
acceleration due to gravity, g = m/s²
height, h = m
E = Kg * m/s² * m
E = kg m² / s²
Comparing both units, we can tell that they are the same.
Acetic acid has the molecular formula CH3COOH. How many atoms of oxygen are there in 60 grams of acetic acid?
Answers
There are approximately 1.203 × 10^24 atoms of oxygen in 60 grams of acetic acid.
To determine the number of atoms of oxygen in 60 grams of acetic acid (CH3COOH), we need to consider the molar mass and the molecular formula of acetic acid.
The molar mass of acetic acid can be calculated by summing the atomic masses of each element in its molecular formula. The atomic masses of carbon (C), hydrogen (H), and oxygen (O) are approximately 12.01 g/mol, 1.01 g/mol, and 16.00 g/mol, respectively.
Molar mass of CH3COOH = (1 × 12.01 g/mol) + (4 × 1.01 g/mol) + (2 × 16.00 g/mol) + 1.01 g/mol
= 60.05 g/mol
Now, we can calculate the number of moles of acetic acid in 60 grams using the molar mass:
Number of moles = Mass / Molar mass
= 60 g / 60.05 g/mol
≈ 0.999 moles
From the molecular formula of acetic acid, we can see that there are two atoms of oxygen in each molecule.
Therefore, the number of atoms of oxygen in 60 grams of acetic acid can be calculated by multiplying the number of moles by the Avogadro's number, which represents the number of particles (atoms, molecules, or ions) in one mole of a substance. Avogadro's number is approximately 6.022 × 10^23 particles/mol.
Number of atoms of oxygen = Number of moles × Avogadro's number × Number of oxygen atoms in one molecule
= 0.999 moles × 6.022 × 10^23 particles/mol × 2
≈ 1.203 × 10^24 atoms
For more such questions on acetic acid. visit:
https://brainly.com/question/15231908
#SPJ8
The titration of a 20.0- mL sample of an H2SO4 solution of unknown concentration requires 22.47 mL of a 0.160 M KOH solution to reach the equivalence point.
What is the concentration of the unknown H2SO4 solution?
I am getting 0.899 no matter what I way I do it and the answer is not being accepted. What is the correct answer?
Answers
When 20.0mL of sulfuric acid titrated with 22.47 mL of a 0.160 M Potassium hydroxide solution, then the calculated molarity comes out to be 0.18M.
What is titration?Titration is a technique by which we know the concentration of unknown solution using titration of this solution with solution whose concentration is known. To know the end point we use phenolphthalein as indicator. End point is a point where completion of reaction happen.
Mathematically,
moles or sulfuric acid =moles of potassium hydroxide solution
\(M_{1} V_{1} =M_{2} V_{2}\)
\(M_{1}\)= Molarity of sulfuric acid
\(V_{2}\)= Volume of sulfuric acid
\(M_{2}\)= Molarity of potassium hydroxide solution
\(V_{2}\)= Volume of potassium hydroxide solution
Substituting all values
\(M_{1}\)= 0.160 M ×22.47 mL)÷20.0- mL
=0.18M
Thus the molarity if sulfuric acid is 0.18M
To know more about titration, here:
https://brainly.com/question/13307013
#SPJ1
Example of change in substance
Answers
The original substance has undergone a transformation into a new substance with different properties, indicating a change in the chemical composition of the material.
An example of a change in substance is the process of combustion. When a substance, such as wood, is burned, it undergoes a chemical reaction with oxygen in the air, which produces a new substance: carbon dioxide gas, water vapor, and ash. This change in the chemical composition of the wood means that it has transformed into a completely new substance with different physical and chemical properties.
Another example is the process of electrolysis, where an electric current is passed through a solution containing ions. This can cause a chemical reaction to occur, resulting in the breakdown of the original substance into its component parts or the formation of a new substance.
for more questions on transformation
https://brainly.com/question/29713522
#SPJ11
Using the measurements in the table, determine which unidentified metal has the lowest density?

Answers
Answer:
Metal C
Explanation:
Density = mass (g)/ volume (mL, which is the same thing as cm cubed)
Divide mass by volume for each metal, the metal with the lowest value (metal C) is your answer.
Answer:
IT IS
NOT. I REPEAT - IT IS NOT> D
Explanation:
i got it wrong :(
Neutral atoms of the same element can differ in their number of
A. neutrons
B. electrons
C. protons
Answers
Answer:
a. neutron
Explanation:
A neutral atom has the same number of protons and electrons because in order for it to be a neutral atom the charges must be the same.
a neutron has no charge therefore it can be different then the number of protons and electrons in a neutral atom as it won't affect the charge.
Light shining on solar cells produces a current that charges a solar calculator. Which model of light does this example support? OA. It supports the particle model. OB. It supports the wave model. OC. It supports the interference model. OD. It supports the diffraction model. SUBMIT Light shining on solar cells produces a current that charges a solar calculator . Which model of light does this example support ? OA . It supports the particle model . OB . It supports the wave model . OC . It supports the interference model . OD . It supports the diffraction model . SUBMIT
Answers
The model of light does the example supports is the particle model.
What is the wave-particle duality model of light?The wave-particle duality model of light is a model which suggests that light behaves both as a [article and as a wave.
The wave-like property is seen in its properties such as reflection, refraction, diffraction, etc.
The particle nature of light is seen in the photoelectric effect and the Compton effect.
Light shining on solar cells produces a current that charges a solar calculator is an example of the photoelectric effect.
Therefore, the model of light does this example support is the particle model.
Learn more about wave-particle model of light at: https://brainly.com/question/14264284
#SPJ1
11. (2 pts) Sodium Hydroxide, is also known as lye and was a critical component in
homemade soap. Now it is a commonly used drain cleaner because it chemically reacts
with fats (the typical cause of a clog) to form a soap that can be swept down the drain.
What is the molarity of 5.00 g Sodium Hydroxide in 750.0 mL of solution?
Answers
Answer:
0.167M
Explanation:
Molarity, M, is an unit of concentration in chemistry defined as the ratio between moles of solute (NaOH in this case) and volume of the solution in liters.
To find molarity of 5.00 g Sodium Hydroxide in 750.0 mL of solution we need to convert mass of NaOH to moles (Using its molar mass: 40g/mol) and the mililiters of solution to liters (1L = 1000mL), thus:
Moles NaOH = 5.00g × (1mol/ 40g) = 0.125 moles NaOH = Moles solute
Liters solution = 750.0mL × (1L / 1000mL) = 0.7500L solution
And molariy is:
0.125 moles NaOH / 0.7500L solution =
0.167M
Some insects, such as water striders, are able to walk on water. In one or two sentences, make and justify a conjecture about a property of water that would allow the insect to walk on it.(2 points) SHORT ANSWER
Answers
Insects can walk on water due to its surface tension, which is something like 0.07 J/m^2.
So water (and most liquids) have a property called surface tension, which is defined as the amount of energy that you need to increase the surface per area unit of the liquid volume.
Thus, if the insect has smaller energy than that, it would not change the surface of the liquid, allowing it to walk on the water's surface.
If you want to learn more about surface tension, you can read:
https://brainly.com/question/11348644
Answer: They are able to walk on water because of surface tension. Liquid molecules are held together by electrostatic forces. Molecules on the surface of the water are attracted to surrounding molecules and tension is created as the molecules get pulled down. This allows them to distribute their body weight over the water’s surface without breaking through that force.
Explanation:
What is the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C? A) 4.48 x 10¹¹ atm B) 2.24 x 10⁰ atm C) 1.12 x 10³ atm D) 2.24 x 10³ atm
Answers
The pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C is 2.24 × 10⁰ atm.
How to calculate pressure?The pressure of a substance can be calculated using the following formula;
PV = nRT
P = pressureV = volumen = no of molesR = gas law constantT = temperatureAccording to this question, the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C can be calculated as follows:
P × 2 = 0.1 × 0.0821 × 546
2P = 4.48266
P = 2.24 × 10⁰ atm
Learn more about pressure at: https://brainly.com/question/31525061
#SPJ1