Consider the molecular structure for linuron, an herbicide, provided in the questions below. a) What is the electron domain geometry around nitrogen-1? b) What is the hybridization around carbon-1? c) What are the ideal bond angles > around oxygen-1? d) Which hybrid orbitals overlap to form the sigma bond between oxygen-1 and nitrogen-2? e) How many pi bonds are in the molecule?
Answers
Answer:
a)Electron domain geometry around nitrogen-1 is tetrahedral
b)Hybridization around carbon-1 is sp2
c)The ideal bond angles around oxygen-1 are 120 degrees.
d)Hybrid orbitals overlapping to form the sigma bond between oxygen-1 and nitrogen-2 is sp2 hybrid orbitals from carbon-1 and nitrogen-2
e)There are no pi bonds in the molecule.
Explanation:
a) Electron domain geometry around nitrogen-1 is tetrahedral.The molecular structure of linuron is as follows: There are three carbon atoms in a row. The terminal carbon atom is linked to a methyl group and a chlorine atom. The carbon atom next to it is linked to the nitrogen atom in the herbicide. The third carbon atom is linked to two oxygen atoms, with one of them being a hydroxyl group.
b) Hybridization around carbon-1 is sp2.The carbon atom adjacent to the nitrogen atom is known as carbon-1. This carbon atom is joined to three other atoms. It has an sp2 hybridization since it has three regions of electron density.
c) The ideal bond angles around oxygen-1 are 120 degrees.Bond angles are the angles between two adjacent lines in a Lewis structure. Because oxygen-1 is linked to two other atoms, it has a bent geometry. Its ideal bond angle is 120 degrees.
d) Hybrid orbitals overlapping to form the sigma bond between oxygen-1 and nitrogen-2 is sp2 hybrid orbitals from carbon-1 and nitrogen-2.The sigma bond is the strongest type of covalent bond. Sigma bonds are created when the overlapping orbitals are arranged in a straight line. The sigma bond between oxygen-1 and nitrogen-2 is formed by the overlap of sp2 hybrid orbitals from carbon-1 and nitrogen-2.
e) There are no pi bonds in the molecule.There are no pi bonds in the molecule because all of the bonds are sigma bonds. The molecule consists of single bonds only.
To know more tetrahedral. about refer here: https://brainly.com/question/18612295#
#SPJ11
Related Questions
help please! asap!
Which of the following experiments could you perform to investigate the effect temperature has on the volume of a gas? ( AKS 5b DOK 2)
A.
Measure the pressure of the air in a tire on a cold day, a warm day, and a hot day.
B.
Measure the volume of a balloon filled with room temperature air. Place the balloon in a freezer for an hour and then measure its volume again.
C.
Stretch a balloon over the top of a beaker filled with baking soda and vinegar and observe how the balloon changes volume.
D.
Inflate one balloon to a large volume and another balloon to a small volume. Measure the temperature of the air released from both balloons.
Answers
Answer:A
Explanation:
Answer:
m
Explanation:
Melting and boiling point of water is high?
Answers
Answer:
Yes
Explanation:
Why melting and boiling point of water is high?
The reason for the high melting and boiling temperatures is the hydrogen bonding between water molecules that causes them to stick together and to resist being pulled apart which is what happens when ice melts and water boils to become a gas
Answer:
the temperature in the hydrogen bonding between water molecules that causes them to stick together which happens when ice melts and water boil to become a gas
Help what's the answer?

Answers
According to the equation, one molecule of H₂ reacts for every two molecules of C₂H₄ that respond at the particle level. One molecule of C₂H₆ is created when these molecules join.
According to the equation, 1 mole of H₂ reacts for every 1 mole of C₂H₄ that does. To create 1 mole of C₂H₆, these reactants must be combined in a ratio of 1:1.
The balanced equation for the chemical reaction is:
\(C_2H_4_(g_) + H_2_(g_) -- > C_2H_6_(g_)\)
This equation illustrates how ethene (C₂H₄) and hydrogen gas (H₂) combine to create ethane. (C₂H₆). The stoichiometric coefficients in the equation denote the mole ratios of the reactants and products in the balanced equation. They are represented by the coefficients in the equation.
According to the equation, one molecule of H₂ reacts for every two molecules of C₂H₄ that respond at the particle level. One molecule of C₂H₆ is created when these molecules join.
According to the equation, 1 mole of H₂ reacts for every 1 mole of C₂H₄ that does. To create 1 mole of C₂H₆, these reactants must be combined in a ratio of 1:1.
In general, the balanced equation and its coefficients offer crucial details about the proportions of reactants and products engaged in the chemical reaction.
Chemical processes are modeled by chemical equations. The proportions of the reactants and products engaged in a chemical reaction are displayed in a balanced chemical equation.
The reactant's and products' mole ratios, which show the relative amounts of each substance engaged in the reaction, are represented by the coefficients in the balanced equation.
The mass conservation principle, says that mass is neither produced nor destroyed in a chemical reaction and experimental data are used to determine these coefficients.
learn more about moles here
https://brainly.com/question/29367909
#SPJ1
14. The atoms of element X contains nineteen electrons. With which of the following elements will the chemistry of Z be similar? a Aluminum b) Bromine c) Lithium d) Magnesium
Answers
First of all, Z is unknown. I hope it is a mistake.
Now, it is given that the element X has nineteen electrons. This proves that X is actually Potassium.
As per the periodic table, both Potassium and Lithium belongs to group 1 as their valency is 1 because of the presence of only one electron in the outermost shell of electrons i.e., they lose an electron during a chemical reaction to form a stable compound. Furthermore, both are metallic.
Magnesium belongs to group 2 and hence its valency is two, which is different from potassium though it is metallic. Similiarly, bromine belongs to group 17 and gains one electron during a reaction in contrast to potassium.
( No internal links available for reference. For clarification, check the periodic table).
HELP ME PLSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSSS

Answers
Explanation:
burning fossil fuels adds carbon dioxide, and photosynthesis removes it
The pathway to release of neurohormones follows a series of steps. Arrange the phrases from left to right to create the correct pathway for the neurohormone ADH from synthesis to release.1) ADH synthesis in supraoptic neurons2) Transport in tract in infundibulum3) Storage in axon termini in posterior pituitary4) Action potentials arrive at the axon terminals
Answers
The regular practise of driving a car to school would be the daily action that would pollute the environment.
This phrase is used to describe all human activities that harm the environment we live in by making it unsafe for the living things that inhabit it. The smoke from exhaust, which is brought on by the burning of gasoline, would be the main source of pollution. It has an adverse impact on the ecosystem because it taints the air, making it hazardous for humans to breathe. It can cause respiratory illnesses in people and other types of poisoning, which has an effect on our health By choosing another mode of transportation or by walking to school, this issue could be lessened.
Learn more about Pollution here:
https://brainly.com/question/28788430
#SPJ4
Q-1 Analysis of sample of polyacrylonitrile shows there are six lengths of chains, with the following number of chains of each length. (Class work) a- Determine the number average molecular weight and degree of polymerization. b. Determine the weight average molecular weight and degree of polymerization
Answers
To determine the number average molecular weight and degree of polymerization, we need to use the following formula: Number average molecular weight (Mn) = ΣNiMi / ΣNi
To find the number average molecular weight and degree of polymerization, we need to first calculate the molecular weight of each chain length. Let's assume the following values for the molecular weights of each chain length: Using the formula above, we can calculate the number average molecular weight as follows: Mn = (1x1000) + (2x2000) + (3x3000) + (4x4000) + (5x5000) + (6x6000) / (1+2+3+4+5+6) Mn = 3100 g/mol
Therefore, the degree of polymerization is an average of the DP values calculated above: DP = (10+20+30+40+50+60) / 6 = 35 To determine the weight average molecular weight and degree of polymerization, we need to use the following formula: Weight average molecular weight (Mw) = ΣNiMi2 / ΣNiMi Using the same molecular weight values as before, we can calculate the weight average molecular weight as follows:
Mw = (1x10002) + (2x20002) + (3x30002) + (4x40002) + (5x50002) + (6x60002) / (1x1000) + (2x2000) + (3x3000) + (4x4000) + (5x5000) + (6x6000) Therefore, the weight average molecular weight is 4300 g/mol and the degree of polymerization is 42.
To Know more about molecular visit;
https://brainly.com/question/20030736
#SPJ11
Which statement is correct?
Baking is a chemical change.
Burning is a physical change.
Rusting is a physical change.
Melting is a chemical change.
Answers
Baking is a chemical change when you bake a cake the ingredients go through a chemical change. .
What is chemical change?Chemical Change is defined as the process in which the atoms of one or more substances are rearranged or combined to form a new substance. When a substance undergoes chemical change the chemical properties of the substance changes and it is transformed into a different substance with different chemical composition. Examples are burning of any substance, rusting of iron.
Therefore a chemical change occurs when the molecules that compose two or more substances are rearranged to form a new substance.
Learn more about chemical change here: brainly.com/question/1222323
#SPJ1
Four cats r sitting in a living room which cat has the greatest gravitational potencial energy
Answers
Answer:
Therefore, amongst four cats, the cat which is at the highest position, will have the greatest gravitational potential energy.
Explanation:
The gravitational potential energy is the energy of the system, which is associated with the position (height) of object from the surface of earth in the gravitational field. The formula for gravitational potential energy is given as:
P.E = mgh
where,
P.E = Gravitational Potential Energy
m = mass of object
g = acceleration due to gravity
h = height from the surface of earth
Therefore, amongst four cats, the cat which is at the highest position, will have the greatest gravitational potential energy.
Which of the following statements correctly describe the solubility product constant Ksp for a slightly soluble substance? Select all that apply
a. Ksp depends on the temperature of the solution.
b. Ksp is independent of the concentrations of the ions in solution.
c. The value of Ksp indicates how far a dissolution equilibrium proceeds in favor of dissolved solute.
Answers
The correct statements regarding the solubility product constant (Ksp) for a slightly soluble substance are:
a. Ksp depends on the temperature of the solution.
c. The value of Ksp indicates how far a dissolution equilibrium proceeds in favor of dissolved solute.
What is the solubility product constant?
The solubility product constant, often denoted as Ksp, is a mathematical constant that represents the equilibrium expression for a slightly soluble compound dissolving in a solvent to form ions. It quantitatively describes the solubility of the compound in a particular solvent.
a. Ksp is influenced by changes in temperature in a solution:
The solubility product constant, Ksp, is influenced by temperature. In general, as temperature increases, the solubility of most substances also increases, leading to a higher value of Ksp. However, this relationship may not hold true for all substances.
b. Ksp is independent of the concentrations of dissolved the ions in solution:
This statement is incorrect. The solubility product constant, Ksp, is directly related to the concentrations of the ions in solution at equilibrium. The value of Ksp is determined by the ratio of the concentrations of the dissolved ions raised to the power of their respective stoichiometric coefficients.
c. The magnitude of Ksp reflects the extent to which a dissolution equilibrium favors the production of dissolved solute.:
This statement is correct. The magnitude of the solubility product constant, Ksp, provides information about the extent to which a dissolution equilibrium proceeds. A higher value of Ksp indicates that the equilibrium favors the formation of dissolved solute and that the substance is more soluble. Conversely, a lower value of Ksp suggests limited solubility and a shift towards the solid phase.
Therefore, the correct statements are
a. Ksp is influenced by changes in temperature in a solution and c. The value of Ksp provides information about the extent to which a dissolution equilibrium proceeds in favor of dissolved solute.
To learn more about the solubility product constant from the given link
brainly.com/question/29350479
#SPJ4
You must apply a force to put an object in motion or ________an object from moving.
(answer the blank)
Answers
Answer:
any push or pull that causes an object to move, stop, or change speed or direction
Description:
zayn your my enemy
Which of the following represent a mole ratio between silver nitrate and appper(II) nitrate in the following reaction: 2AgNO3 + Cu --> Cu(NO3)2 + 2Af
Answers
There is no direct involvement of \(Cu(NO_3)_2\) in the mole ratio calculation as it is not a reactant with \(AgNO_3.\)
The balanced chemical equation for the given reaction is:
\(2AgNO_3 + Cu -- > Cu(NO_3)_2 + 2Ag\)
According to this equation, the mole ratio between \(AgNO_3\) and Cu is 2:1, which means that for every 2 moles of \(AgNO_3\) used, 1 mole of Cu is consumed.
There is no direct mole ratio between \(AgNO_3\) and \(Cu(NO_3)_2\) or between \(AgNO_3\) and Ag. However, we can calculate the mole ratio between \(AgNO_3\) and Ag using the stoichiometric coefficients in the balanced equation.
For every 2 moles of \(AgNO_3\) used, 2 moles of Ag are produced. Therefore, the mole ratio between \(AgNO_3\) and Ag is 2:2 or simply 1:1.
To know more about reaction here
https://brainly.com/question/11231920
#SPJ1
Metals and nonmetals gain stability by losing or gaining electrons to form ions with stable valence electron configurations. This type of bonding is called ______ bonding.
Answers
Metals and nonmetals gain stability by losing or gaining electrons to form ions with stable valence electron configurations. This type of bonding is called ionic bonding.
Ionic bonding is a type of chemical bonding where ions are formed from the transfer of electrons between a metal and nonmetal. Metals tend to lose electrons to become positively charged cations, while nonmetals tend to gain electrons to become negatively charged anions.
The resulting oppositely charged ions attract each other and form a crystal lattice structure, creating an ionic bond. This type of bonding typically occurs between elements with a large electronegativity difference, such as metals and nonmetals, and results in the formation of compounds known as ionic compounds or salts.
Ionic compounds have high melting and boiling points, are typically solid at room temperature, and are electrically conductive when molten or dissolved in water.
For more questions like Ionic bonding click the link below:
https://brainly.com/question/11527546
#SPJ11
A benzene-toluene mixture is to distilled in a simple batch distillation column. If the mixt re contains 60% benzene and 40% toluene, what will be the boiling point of mixture if it is to be distilled at 2 atm? (A) 90 B) 122 115 (D) 120
Answers
To determine the boiling point of the benzene-toluene mixture at 2 atm, we need to consider the vapor-liquid equilibrium of the mixture.
The boiling point of a liquid corresponds to the temperature at which its vapor pressure is equal to the external pressure. Given that the mixture contains 60% benzene and 40% toluene, we can assume ideal behavior and calculate the vapor pressure of each component using Raoult's law: P_benzene = X_benzene * P°_benzene; P_toluene = X_toluene * P°_toluene, Where X_benzene and X_toluene are the mole fractions of benzene and toluene, respectively, and P°_benzene and P°_toluene are the vapor pressures of pure benzene and toluene at the given temperature. Assuming ideal behavior, the total vapor pressure of the mixture is given by: P_total = P_benzene + P_toluene.
Since the mixture is distilled at 2 atm, we can set up the equation: P_total = 2 atm. By substituting the known values and solving the equation, we can determine the boiling point of the mixture. Note: The given answer options (90, 122, 115, 120) do not correspond to the boiling points in degrees Celsius. It is necessary to convert the obtained boiling point from Kelvin to Celsius to match the provided answer options.
To learn more about boiling point click here: brainly.com/question/2153588
#SPJ11
Fe+H2SO4------>????????????
Answers
equation: Iron + Sulfuric acid → Iron (II) sulfate + hydrogen gas
Type of Chemical Reaction: For this reaction we have a single displacement reaction.
Note that this is the chemical reaction that takes place when diluted Sulfuric acid reacts with iron. If you have concentrated Sulfuric acid you will have a different equation.
Calculate the volume of an object that has a mass of 20.00 g and a density of 2.45 g/cm^3.
Answers
Answer:
2450000 gram
Explanation:
v= m × ρ= 1 cubic meter × 2.45 gram/cubic centimeter= 1000000 cubic centimeter × 2.45 gram/cubic centimeter= 2450000 gram
Explanation:
v=m over D
mass=20.oog
Density=2.45g
20.00-2.45
ans is 17.46
Neutral atoms of all isotopes of a given element have the same
A) number of protons and neutrons
B) number of neutrons
C) number of electrons
D) mass numbers
E) masses
Answers
Neutral atoms of all isotopes of a given element have the same number of protons and neutrons.
All the isotopes of the same element consists of the same number of protons, which defines the element.
Depending on the neutron count, different isotopes can exist . This is because electrons are negatively charged and they can counterbalance the positive charge of protons. Also, they are present in equal numbers in neutral atoms of the same element.
Mass number is formed from the addition of protons and neutrons, which varies between isotopes. An isotope's mass can differ between different isotopes since it depends on its mass number and the masses of the particles that it is formed from.
However, the masses of various isotopes of the same element are extremely close. Hence, this can be challenging to distinguish without the help of specialized tools.
Thus, option (a) is correct.
To know more about neutral atoms here
https://brainly.com/question/30485099
#SPJ4
Not a timed or graded assignment. Quick answering will get an amazing review, thank you :)

Answers
This law states that matter can neither be created nor destroyed in an ordinary chemical reaction, but can change from one form to another.
What this implies is that when two elements or compound combine as a reactant to form a product, there's no loss of matter and the mass of the reactants must be equal to the mass of the product.
Given that
AlBr₃ + K₂SO₄ → KBr + Al₂(SO₄)₃
If we look closely at the above reaction, we would see that aluminuim is not balanced, potassium is also not balanced, bromine is not balance as well as sulphur and oxygen.
Let's put two moles attached to the AlBr₂ and three moles of K₂SO₄
2AlBr₃ + 3K₂SO₄ →
This would give us
6KBr + Al₂(SO₄)₃
If we add the two equations together,
2AlBr₃ + 3K₂SO₄ → 6KBr + Al₂(SO₄)₃
From the above, we have
2 atoms of Al on the reactant side and 2 atoms of Al on the product side
6 atoms of Br on the reactant side and 6 atoms of Br on the product side
6 atoms of K on the reactant side and 6 atoms of K on the product side
3 atoms of S on the reactant side and 3 atoms of S on the product side
12 atoms of O on the reactant side and 12 atoms of O on the product side
After hockey practice, Carissa and Keenan were playing a game where they were pushing some objects to get them to crash. They were using a cone and two different pucks—a black one with more mass for Crash 1 and a blue one with less mass for Crash 2. They want to know what happened to the cone. Use the information from the diagram to answer. In which crash did the cone experience a stronger force? How do you know?
Answers
The crash where the cone experience a stronger force is option D because: Crash 1: the force on the black hockey puck was stronger in this crash, so the force on the cone was also stronger.
Does it take a stronger force to slow something down?The ball is drawn back to Earth by gravitational force. The ball returns to Earth as a result of friction. The ball is forced back toward Earth by magnetic force.
A puck's velocity changes when a player makes contact with it when it is still. He causes the puck to speed up, in other terms. The hockey stick's force, which causes the acceleration, is responsible. The velocity grows as long as this force is in motion.
Therefore, the force applied to an object must be larger than what is required for a progressive slowing down if the object must be slowed down quickly. For instance, a bicycle's brakes will slow or stop it more quickly the more force is given to it.
Learn more about Force from
https://brainly.com/question/12785175
#SPJ1
See full question below
After hockey practice, Carissa and Keenan were playing a game where they were pushing some objects to get them to crash. They were using a cone and two different pucks—a black one with more mass for Crash 1 and a blue one with less mass for Crash 2. They want to know what happened to the cone.
Use the information from the diagram to answer.
In which crash did the cone experience a stronger force? How do you know?
answer choices
There was no force on the cone. In both crashes, only the hockey puck experienced a force.
The diagram doesn’t tell you anything about the force on the cone. It only gives information about the force on the pucks.
It was the same force in both crashes; the hockey puck changed speed by the same amount in each crash, so the force on the cone was the same each time.
Crash 1; the force on the black hockey puck was stronger in this crash, so the force on the cone was also stronger.

How many moles of carbon would 50.0 g of carbon represent?
Answers
Answer:
600 grams
Explanation:
The value of the mole is equal to the number of atoms in exactly 12 grams
12x50=600
Convert 7.80 quarts into milliliters.
Show your work, include units with every number, and round your answer to the
correct number of significant figures.
Answers
Explanation:
1 quart = 946.35 milliliters
Therefore, 7.80 quarts • 946.35 = 7381.55
Round .55 and it becomes 7382 milliliters
the nitrogen bases are held together in the center of the molecule by
Answers
In the DNA ,the nitrogen bases are held together in the center of the molecule by the hydrogen bond.
The nitrogen bases are held together in the center of the DNA molecule by the hydrogen bond. The hydrogen bond that is the H - bond binds the nitrogen bases in between the two strands of the DNA. There are the two hydrogen bonds that is present in between the A and the T and the three hydrogen bond in between the G and the C.
The hydrogen bond in the DNA held together the nitrogen bases in the center of the molecule.
This question is incomplete, the complete question is :
In the DNA ,the nitrogen bases are held together in the center of the molecule by ?
To learn more about DNA here
https://brainly.com/question/11871151
#SPJ4
Consider the reactions below. Which of the following correctly identifies the coordinate complex? Select the correct answer below: O SO3 in 02- + SO3 + S02 02- in 02- + S03 +502 BH3 in (CH3), S +BH3 → H3BS(CH3), O Becl in BeCl2 +201 Beci - NA MODE INSTRUCT
Answers
Out of the given reactions, the correct identification of the coordinate complex is BH3 in (CH3)2S + BH3 → H3BS(CH3)2. In this reaction, BH3 acts as a Lewis acid and coordinates with the lone pair of electrons present on the S atom in (CH3)2S to form a coordinate complex.
The BH3 molecule is a Lewis acid as it has an incomplete octet and can accept a pair of electrons from a Lewis base. In the other two reactions, there are no coordination complexes formed.
BeCl2 is not involved in the formation of a coordination complex in the given reactions. It is a molecule that exists as a linear shape due to its sp hybridization. The two Cl atoms are directly bonded to the central Be atom through a single bond. BeCl2 is not a Lewis acid as it does not have an incomplete octet and cannot accept a pair of electrons from a Lewis base to form a coordination complex.
In conclusion, the correct identification of the coordinate complex is BH3 in (CH3)2S + BH3 → H3BS(CH3)2, and BeCl2 is not involved in the formation of a coordination complex in the given reactions.
To know more about Lewis acid visit:
https://brainly.com/question/15209937
#SPJ11
Answer:
BeCl2−4 in BeCl2 + 2Cl → BeCl2−4
Explanation:
In a Lewis acid-base reaction, the coordinate complex is the compound that is generated by the formation of coordinate covalent bond(s) between the Lewis acid and the Lewis base.
Cassandra builds a galvanic cell using a zinc electrode immersed in an aqueous Zn(NO3)2 solution and an copper electrode immersed in an aqueous CuCl2 solution at 298 K. Which species is produced at the anode
Answers
In the galvanic cell that Cassandra builds with a zinc electrode in a Zn(NO3)2 solution and a copper electrode in a CuCl2 solution at 298 K, the species produced at the anode is Zn2+.
1. In a galvanic cell, the anode is where oxidation occurs.
2. The zinc electrode (Zn) will act as the anode, as it has a lower reduction potential compared to the copper electrode (Cu).
3. During the oxidation process at the anode, the zinc electrode loses electrons and becomes Zn2+ ions, which dissolve into the aqueous Zn(NO3)2 solution.
4. Therefore, the species produced at the anode in this galvanic cell is Zn2+.
For more information on galvanic cell involving electrodes https://brainly.com/question/29765093
#SPJ11
Why do you not come to thermal equilibrium on a cold day
Answers
Answer:
i have no idea i ma doing this for the point good luck though
Explanation:
What are the phases for the products of reaction I-2?CuSO4 5H2O (s) —> CuSO4 + 5H2OA. CuSO4 (aq) + 5H2O (l)B. CuSO4 (s) + 5H2O (l)C. CuSO4 (s) + 5H2O (aq)D. Ca+2H2O→ Ca(OH)2+H2
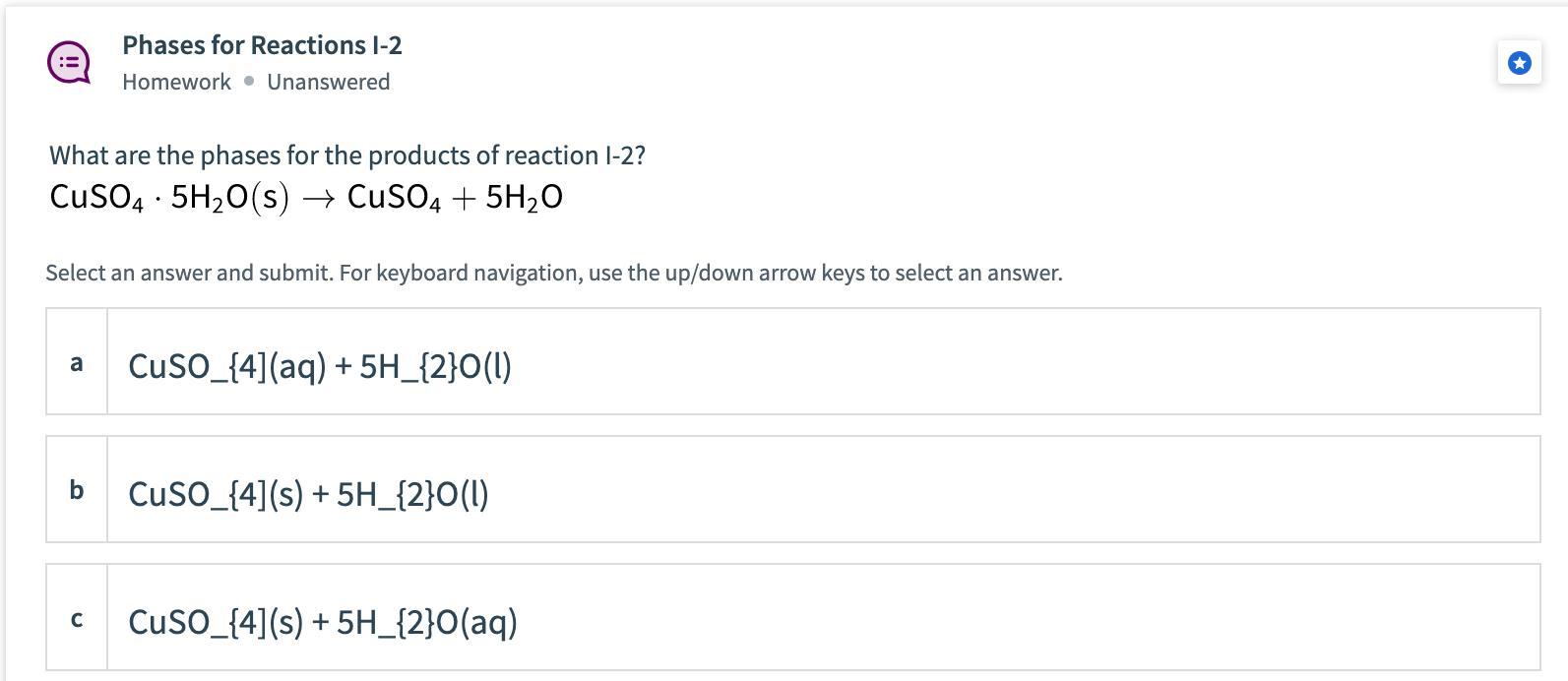
Answers
According to the given reaction, the phase of the salt, CuSO₄, is solid, it means that its subscript is (s).
The phase of water, H₂O, is liquid, it means that its subscripts is (l).
It means that the answer is B.
after adding the grignard solution to your flask, you washed your syringe with acetone, which cause the syringe to heat up as a white solid formed. why did the syringe heat up and what is the chemical structure of the white solid formed?
Answers
The syringe heated up due to the formation of side product bromobenzene which leads to the whitish appearance.
What is grignard reagent and what is the product formed which heated up the syringe?Grignard reagent is highly reactive chemical reagent depicted as RMgX and is highly used compound in organic chemistry.In grignard reagent the formula RMgX , where in R is the alkyl group, Mg is magnesium and X is halogen.It is specifically kept away from water because in the presence of the water it reacts vigorously and form hydrocarbon.In here after the addition of grignard reagent to the flask , the syringe is washed off with acetone, causing the syringe heat up due to the formation of bromobenzene.Bromobenze immediately heats up the syringe causing the whitish appearance .To know more about grignard reagent visit:
https://brainly.com/question/14702056
#SPJ4
Consider the chemical equation.
CuCl2 + 2NaNO3 Right arrow. Cu(NO3)2 + 2NaCl
What is the percent yield of NaCl if 31.0 g of CuCl2 reacts with excess NaNO3 to produce 21.2 g of NaCl?
Use Percent yield equals StartFraction actual yield over theoretical yield EndFraction times 100..
49.7%
58.4%
63.6%
78.7%
Answers
Percent yield = 78.7% , the correct answer is D) 78.7%, which represents the percent yield of NaCl in the reaction.
To calculate the percent yield of NaCl in the given chemical equation, we need to compare the actual yield of NaCl with the theoretical yield. The theoretical yield is the amount of NaCl that would be produced if the reaction went to completion based on stoichiometry.
First, we need to determine the theoretical yield of NaCl. By examining the balanced equation, we can see that the stoichiometric ratio between CuCl2 and NaCl is 1:2. This means that for every 1 mole of CuCl2, 2 moles of NaCl are produced.
Step 1: Convert the mass of CuCl2 to moles using its molar mass.
Molar mass of CuCl2 = 63.55 g/mol (atomic mass of Cu) + 2 × 35.45 g/mol (atomic mass of Cl)
Molar mass of CuCl2 = 134.45 g/mol
Moles of CuCl2 = 31.0 g / 134.45 g/mol ≈ 0.231 mol
Step 2: Use the stoichiometry to calculate the theoretical yield of NaCl.
Since the stoichiometric ratio between CuCl2 and NaCl is 1:2, the moles of NaCl produced will be twice the moles of CuCl2.
Moles of NaCl (theoretical) = 2 × 0.231 mol = 0.462 mol
Step 3: Convert the moles of NaCl to grams using its molar mass.
Molar mass of NaCl = 22.99 g/mol (atomic mass of Na) + 35.45 g/mol (atomic mass of Cl)
Molar mass of NaCl = 58.44 g/mol
Theoretical yield of NaCl = 0.462 mol × 58.44 g/mol ≈ 26.96 g
Now, we can calculate the percent yield using the formula:
Percent yield = (Actual yield / Theoretical yield) × 100
Percent yield = (21.2 g / 26.96 g) × 100 ≈ 78.7%
Option D
For more such questions on Percent yield visit:
https://brainly.com/question/14714924
#SPJ8
THE FIRST PERSON TO ANSWER GETS BRAINLIST NO NEED TO EXPLAIN! SUPER EASY!!!!!!!!

Answers
Answer: the answer is true
Explanation:
what process separates pigments from a plant extract?
Answers
The process that separates pigments from a plant extract is known as chromatography.
This technique involves the separation of different components of a mixture based on their chemical properties and interactions with a stationary phase and a mobile phase. In the case of plant pigments, the stationary phase can be a paper or a thin layer of silica gel or alumina, and the mobile phase is typically a solvent that is able to dissolve the pigments.
When the plant extract is applied to the stationary phase, the pigments will migrate through the mobile phase at different rates depending on their chemical properties, such as their polarity and size. This results in the separation of the pigments into distinct bands or spots on the stationary phase.
There are several types of chromatography techniques that can be used to separate pigments from a plant extract, including paper chromatography, thin-layer chromatography, and high-performance liquid chromatography. These techniques vary in their level of sensitivity, resolution, and speed, but they all rely on the principles of chromatography to isolate and identify the different pigments present in the plant extract.
For more such questions on chromatography
https://brainly.com/question/1394204
#SPJ11