The renal control mechanism of restoring the acid-base balance is accomplished through which process?
Answers
Re absorption of H\(CO_{3}\) and excretion of H+ restores acid-base balance through the renal control mechanisms.
What is renal mechanism?
The renal system consists of the kidney, ureters, and therefore the urethra. the general function of the system filters approximately 200 liters of fluid a day from renal blood flow which allows for toxins, metabolic waste products, and excess ion to be excreted while keeping essential substances within the blood
Why is renal function important?
Renal function is important for homeostasis. The kidneys play important pleiotropic roles including removal of metabolic waste products and maintenance of water–electrolyte balance and vital sign . Early diagnosis of renal dysfunction and institution of appropriate therapy are vital to survival.
Learn more about renal control:
brainly.com/question/23945041
#SPJ4
Related Questions
Suggest 2 ways that chemical pollutants could be carried far from their sources?
Answers
Answer:
1.Transport through surface water
2.Transprt through soil
Explanation:
I learned it from my 7th- grade science teacher and suprisingly I still t it. Hope this helps.
What is the energy change per gram of ice when an iceberg composed of pure water, cp = 2.06 j/(gk, is heated from -25°c to -15°c?
Answers
Answer:
\(Q=20.6\frac{J}{g}\)
Explanation:
Hello there!
In this case, according to the equation for the calculation of the heat during a heating process:
\(Q=mC(T_f-T_i)\)
It is possible to compute it per gram of ice by just removing m from the equation by dividing at both sides. Next we plug in the given specific heat and the final and initial temperatures to obtain:
\(Q=2.06\frac{J}{g\°C}[-15\°C-(-25\°C)] \\\\Q=20.6\frac{J}{g}\)
Best regards!
A sample of nitrogen occupies 10.0 liters at 25oC , what would be the new volume at 20oC?
Answers
Apply Charles law
\(\\ \bf\bull\rightarrowtail V1T2=V_2T1\)
\(\\ \bf\bull\rightarrowtail 10(20)=25V_2\)
\(\\ \bf\bull\rightarrowtail 200=25V_2\)
\(\\ \bf\bull\rightarrowtail V_2=8L\)
Answer:
hope it's helpful to you

as the temperature of a gas decreases is volume
Answers
Answer:
it's volume also decrease
Two gases, helium and X, are released from one end of an evacuated long cylinder at the same time. If it takes the helium atoms 4 times faster than gas X to effuse from one end of the cylinder to the other end, what is gas X
Answers
If helium atoms effuse 4 times faster than gas X, then the ratio of their rates of effusion is 4:1. This means that the molar mass ratio of helium to gas X is (1/4)²= 1/16.
Helium atoms are atoms of the chemical element helium, which is a colorless, odorless, and tasteless gas that is the second lightest element in the periodic table. Helium is a noble gas, which means it is chemically inert and does not readily form compounds with other elements. The atomic number of helium is 2, which means it has two protons in its nucleus and two electrons in its outer shell.
Helium atoms have a very low atomic mass and are therefore very light, which makes them useful for a variety of applications, including as a lifting gas in balloons and airships, as a coolant in nuclear reactors and MRI machines, and as a tracer gas in leak detection and other industrial processes. Helium is also important in astrophysics, as it is a key component of stars and plays a role in the process of nuclear fusion.
To learn more about Helium atoms visit here:
brainly.com/question/4945478
#SPJ4
Someone please help me with these anything is helpful thanks

Answers
Explanation:
1 sulfur dioxide 2 monosulfur dinitride 10 nitrogen dioxide
What does the salinity of sea water represent?
the volume of salt in the ocean
the mass of salt in the ocean
the concentration of salt dissolved in the ocean
the concentration of all dissolved chemicals in the ocean
PLS I NEED HELP
Answers
Answer:
the concentration of salt dissolved in the ocean
Explanation:
How many MORE electrons can nitrogen hold in its outer energy level
Answers
Answer:
1Explanation:
Nitrogen has an atomic number of 7
2 in the K shell
7 in the L shell(instead of 8)
8-1=7
Fluorine reacts with copper to form an ionic compound.
Explain, in terms of electrons and electronic structure, what
happens to a fluorine atom when it reacts with copper.
Use the diagram from part (c) to help you to answer this
question.
itens woH
Answers
Answer:
It gains an electron from another atom in reactions, forming a fluoride ion, F -. Note that the atom is called fluorine, but the ion is called fluoride.
Explanation:
According to the electronic configuration of fluorine and copper in the formation of an ionic compound of copper fluoride electron is lost by copper which is gained by fluorine.
Ionic compounds are the compounds which are formed by the transfer of electrons between the atoms which results in the formation of ions.There are two types of ions:anion and cation which are negatively and positively charged respectively.
These are solid and brittle and are good conductors of electricity and heat only in molten state.The transfer of electrons due to the fact that each atom strives to achieve stable electron electronic configuration in doing so it looses or accepts valency as per it's electronic configuration.
In case of copper fluoride, 2 valence electrons of copper are lost which are gained by 2 fluorine atoms resulting in stable electronic configuration of each atom and a stable ionic compound of copper fluoride is formed.
Thus, as per electronic configuration of fluorine and copper in the formation of an ionic compound of copper fluoride electron is lost by copper which is gained by fluorine.
Learn more about ionic compounds,here:
https://brainly.com/question/30420333
#SPJ5
You have 125g of potassium sulfate and 325.6 L of solution. What is the concentration of your solution?
Answers
Answer:
0.0022 concentration
Explanation:
concentration = moles of solute / liters of solution
first off, convert 125g of potassium sulfate into moles. we can do this using conversion factors. the conversion factor we will use is potassium sulfate's molar mass.
125g K2SO4 * ( 1 mol / 174.259) = 0.720 moles potassium sulfate
then, we follow the concentration formula
0.720 moles potassium sulfate / 325.6 L = 0.0022 concentration
Do you have more gravity when your on the ground or in the air
Answers
The gravity force on an object from the Earth is the same regardless of whether the object is surrounded by air .
the Earth has an average gravitational force. Different locations on Earth have gravitational forces that are larger or smaller than average. This is because each location has more or less mass than the average
how to determine percent composition of a mixture of sugar and salt using conductivity
Answers
Measuring the conductivity and comparing it with a calibration curve of known mixtures, you can determine the percent composition of a mixture of sugar and salt.
To determine the percent composition of a mixture of sugar and salt using conductivity, you can follow these steps:
Measure the electrical conductivity: Begin by measuring the electrical conductivity of the mixture. Electrical conductivity is a property that quantifies the ability of a substance to conduct an electric current. In this case, the conductivity of the mixture will be influenced by the presence of sugar and salt.
Conductivity of pure substances: Next, measure the conductivity of pure sugar and pure salt solutions separately. This step is important to establish a baseline and determine the conductivity values of each substance individually.
Prepare calibration solutions: Prepare several calibration solutions by mixing known amounts of sugar and salt in different ratios. For example, you can create solutions with 10% sugar and 90% salt, 20% sugar and 80% salt, and so on. Measure the conductivity of each calibration solution.
Create a calibration curve: Plot a graph with the percent composition of sugar on the x-axis and the corresponding conductivity values on the y-axis. The calibration curve should indicate the relationship between the percentage of sugar in the mixture and the conductivity.
Measure the mixture conductivity: Measure the conductivity of the mixture containing the unknown percentage of sugar and salt. Use the calibration curve to determine the corresponding percent composition of sugar in the mixture.
By comparing the conductivity of the mixture with the calibration curve, you can determine the percentage of sugar in the mixture. This method relies on the fact that different substances have varying conductivities, allowing you to correlate conductivity measurements with the composition of the mixture.
Know more about Conductivity here:
https://brainly.com/question/21496559
#SPJ11
Use the equation below to solve the problem that follows.
2H2 (g) + O2 (g) → 2H2O (g)
When David reacts 13.8 grams of hydrogen gas with excess oxygen, 87.0 grams of water are formed. Calculate his percent yield of water.
Answers
Percent yield = 70%
Further eplanationPercent yield is the comparison of the amount of product obtained from a reaction with the amount you calculated
General formula:
Percent yield = (Actual yield / theoretical yield )x 100%
An actual yield is the amount of product actually produced by the reaction. A theoretical yield is the amount of product that you calculate from the reaction equation according to the product and reactant coefficients
Reaction
2H₂ (g) + O₂ (g) → 2H₂O (g)
mass of H₂O (theoretical) :\(\tt mass=mol\times MW(mol~ratio~H_2O\div H_2=2\div 2)\\\\mass=(\dfrac{2}{2}\times \dfrac{13.8}{2})\times 18~g/mol\\\\mass=124.2~g\)
percent yield\(\tt \%yield=\dfrac{87}{124.2}\times 100\%=\boxed{\bold{70\%}}\)
Ethanol is a:
A. substance
B. heterogeneous mixture
C. homogenous mixture
Answers
Answer: i think it is A
Explanation:
hope this helps!
answer this step by step pls
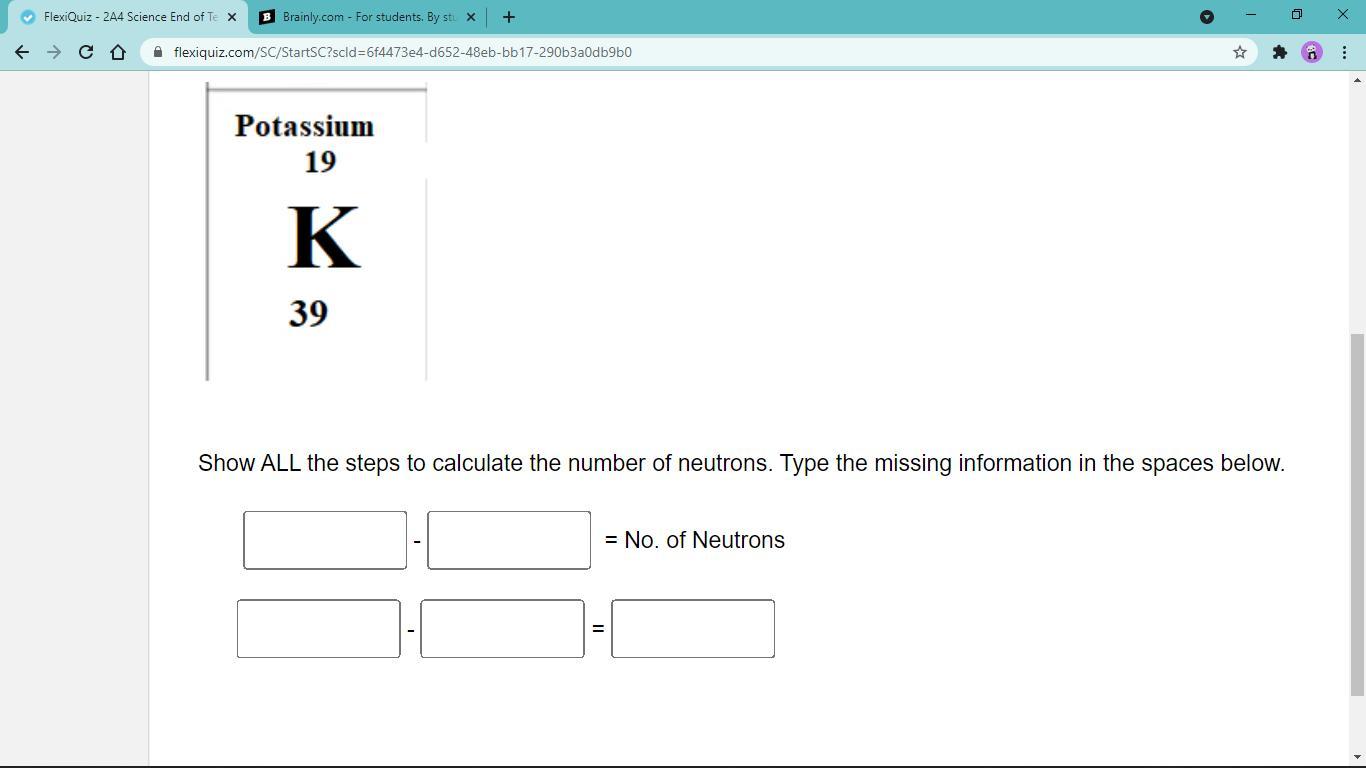
Answers
Answer:
neutrons = mass number - atomic number
. = 39-19 =20
M(s) + 3Ag+(aq) →3Ag(s) + M3+(aq) E° = +2.46 V
Ag+(aq) + e-→Ag(s) E° = +0.80 V
According to the information above, what is the standard reduction potential for the half-reaction M3+(aq) + 3e-→M(s)?
-1.66 V
-0.06 V
0.06 V
1.66 V
Answers
The standard reduction potential for the half-reaction M3+(aq) + 3e- → M(s) can be determined by subtracting the standard reduction potential of the half-reaction Ag+(aq) + e- → Ag(s) from the overall reaction's standard potential.
Given:
E°(overall reaction) = +2.46 V (from the balanced equation)
E°(Ag+(aq) + e- → Ag(s)) = +0.80 V (given)
To find E°(M3+(aq) + 3e- → M(s)):
E°(M3+(aq) + 3e- → M(s)) = E°(overall reaction) - E°(Ag+(aq) + e- → Ag(s))
E°(M3+(aq) + 3e- → M(s)) = +2.46 V - (+0.80 V)
E°(M3+(aq) + 3e- → M(s)) = +1.66 V
Therefore, the standard reduction potential for the half-reaction M3+(aq) + 3e- → M(s) is +1.66 V.
Learn more about standard reduction potential here : brainly.com/question/31868529
#SPJ11
What is the compound name of phosphate ?
Answers
Answer:
Phosphate, any of numerous chemical compounds related to phosphoric acid (H3PO4).
what volume would be occupied by 100.0g of oxygen gas at a pressure of 1.5atm and a temperature of 25c?
Answers
100.0 g of oxygen gas at a pressure of 1.5 atm and a temperature of 25°C would occupy a volume of 49.2 L.
To solve this problem, we can use the Ideal Gas Law, which states:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
We need to rearrange this equation to solve for the volume V:
V = (nRT) / P
where n is the number of moles of the gas, which we can calculate using the molar mass of oxygen gas:
n = m / M
where m is the mass of the gas and M is the molar mass of oxygen gas (32 g/mol).
n = 100.0 g / 32 g/mol = 3.125 mol
Now we can substitute the given values into the equation to find the volume:
V = (nRT) / P
V = (3.125 mol)(0.0821 L·atm/mol·K)(298 K) / 1.5 atm
V = 49.2 L
Therefore, 100.0 g of oxygen gas at a pressure of 1.5 atm and a temperature of 25°C would occupy a volume of 49.2 L.
Click the below link, to learn more about Volume of oxygen:
https://brainly.com/question/28577843
#SPJ11
A molecule is
A a group of atoms bonded by ionic bonds.
B a group of atoms bonded by covalent bonds.
C a group of ions bonded by covalent bonds.
D a group of atoms bonded by metallic bonds.
Answers
Answer:
B.
Explanation:
Atoms make up molecules and ionic bonds cannot make up a molecule because they do not share electrons like in a covalent bond.
In an electrolytic cell, the electrode that acts as a source of electrons to the solution is called the __________; the chemical change that occurs at this electrode is called __________.
Answers
In an electrolytic cell, the electrode that acts as a source of electrons to the solution is called the cathode; the chemical change that occurs at this electrode is called reduction.
Define Electrolyte:-An electrolyte is a material that separates into charged ions when it is in contact with water. Cations are positively charged ions. Anions are ions that are negatively charged. A substance that may conduct an electric current when melted or dissolved in water is known as an electrolyte.
Electrochemical cellThere are three main categories of electrochemical cells. the galvanic cell, the concentration cell, and the electrolytic cell. These cells all share the same four fundamental components. These are the elements
The electrolyte serves as the conduit for current flow between the anode and the cathode. In an aqueous solution, it normally is homogeneous, but in moist soil, the concentration or kind of dissolved compounds may vary locally.The anode, which can conduct electricity and is in contact with the electrolyte, corrodes when it combines with the chemicals in the electrolyte.A metal also contacts the electrolyte at the cathode. It is protected from corrosion rather than corroded.Anode and cathode are connected by the conductor, which also completes the circuit.Learn more about Electrochemical cells here:-
https://brainly.com/question/25749323
#SPJ4
During a flood, a mountain stream is carrying clay, silt, sand, and pebbles. The streambed and particle sizes are shown below. Which sediments will most likely be deposited first when the stream slows down?
Answers
Answer:
Bigger particals first but I would say rocks or pebbles
Explanation:
During flood, when the stream slows down, the clay and sand sediments will deposited first sinc particle size is small.
What is flood?Flood is a natural calamity where, the water reservoirs overflows to the land area and leads to destruction in buildings and houses. The results depends on the speed of water flow.
Flood occurs due to the pressure difference beneath water and due to heavy rain. There are many more evidences, that flood caused significant losses in land areas in many coutries.
The flow of particles with the stream bed depends on their size and weight. The massive particles will only flow and sediment when the stream flows with greater speed. Thus, sand and clay particles sediment first and other heavy particles will flow slowly and deposit later.
To find more on flood, refer here:
https://brainly.com/question/28725721
#SPJ2
23. Under which conditions would carbon dioxide be most soluble in water?
O 1) 10°C and 1 atm
O2) 10°C and 2 atm
3) 20°C and 1 atm
4) 20°C and 2 atm
Answers
Answer:
20°C and 2 atm is correct
Explanation:
What is the control in the experiment?

Answers
Answer:
C. the amount of drug x given to mice
A student is trying to identify an unknown metal X. When he puts it in copper sulphate there is a reaction and red brown pieces of copper fall to the bottom of the test tube. But when he puts metal X into magnesium chloride nothing happens
A) Give two identity of metal X.
B) Out of these two which one is metal X ?
Answers
The unknown metal X is iron metal as it reacts with copper sulfate solution but does not react with magnesium chloride.
What is displacement reaction?Some metals are very reactive while other metals are less reactive or unreactive. When a more reactive metal is added to the solution of a less reactive metal, then the more reactive displaces the less reactive metal from its solution is known as a displacement reaction.
The general form of a single displacement reaction can be represented as:
\(A + BC \longrightarrow B + AC\)
When iron is placed in copper sulfate (CuSO₄) solution then the blue color of the copper sulfate solution turns a red-brown coating of copper metal deposited on the iron.
\(CuSO_4 (aq)+ Fe (s)\longrightarrow FeSO_4 (aq) +Cu(s)\)
Iron lies above the electrochemical series and is more reactive than copper. So it reacts with copper sulfate but does not give any reaction with magnesium chloride.
Learn more about displacement reaction, here:
https://brainly.com/question/3172917
#SPJ1
Hydrogen cyanide, HCN, is the poisonous gas used in the gas chamber. It can be formed by the reaction:
NaCN+H - HCN+Nat
What mass of NaCN, sodium cyanide, is required to make 14.7 L HCN at STP?
Answers
Approximately 29.5 g of NaCN is required to make 14.7 L of HCN at STP.
To solve this problem, we will use the ideal gas law to calculate the number of moles of HCN produced and then use stoichiometry to determine the mass of NaCN required.
First, we need to determine the number of moles of HCN produced using the ideal gas law:
\(PV = nRT\)
where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature.
At STP (standard temperature and pressure), P = 1 atm and T = 273 K. The volume of HCN produced is given as 14.7 L.
Plugging these values into the ideal gas law, we get:
\(n = PV/RT = (1 atm) *(14.7 L)/(0.0821 L atm/mol K * 273 K) = 0.603 mol\)
So, 0.603 mol of HCN is produced.
Now we can use stoichiometry to determine the mass of NaCN required. From the balanced chemical equation:
NaCN + HCl → NaCl + HCN
we can see that 1 mole of NaCN produces 1 mole of HCN.
Therefore, the mass of NaCN required can be calculated as:
mass of NaCN = number of moles of NaCN x molar mass of NaCN
The molar mass of NaCN is 49.01 g/mol.
So, the mass of NaCN required is:
mass of \(NaCN = 0.603 mol * 49.01 g/mol = 29.5 g\)
Therefore, approximately 29.5 g of NaCN is required to make 14.7 L of HCN at STP.
To know more about mole:
https://brainly.com/question/26416088
the speed of a chemical reaction (its reaction time) is the change
Answers
The speed of a chemical reaction, also known as its reaction time, is the change in the concentration of reactants or products over a specific period. This speed can be influenced by factors such as temperature, concentration, and the presence of catalysts. In general, a faster reaction speed indicates a quicker transformation of reactants into products during the chemical reaction.
The speed of a chemical reaction refers to how fast or slow a chemical reaction takes place. It is determined by the rate at which the reactants are consumed and the products are formed. The chemical reaction time can be explained by the rate of collision between the reacting molecules. When two molecules collide with enough energy, they can break their bonds and form new ones, resulting in a chemical reaction. The more collisions that occur in a given amount of time, the faster the chemical reaction will proceed. Therefore, the speed of a chemical reaction is dependent on the frequency and energy of collisions between the reacting molecules.
Learn more about the chemical reactions at https://brainly.com/question/25769000
#SPJ11
Can someone answer please, also please give the steps.

Answers
The volume (in milliliters) of the 2.00 M NaOH solution that can be produced from the reaction is 955 mL
How do i determine the volume of NaOH produced?First, we shall determine the mole of 44.00 grams of Na that reacted. Details below:
Mass of Na = 44.00 grams Molar mass of Na = 22.99 g/mol Mole of Na =?Mole = mass / molar mass
Mole of Na = 44 / 22.99
Mole of Na = 1.91 moles
Next, we shall determine the mole of NaOH obtained from the reaction. Details below:
2Na + 2H₂O -> 2NaOH+ H₂
From the balanced equation above,
2 moles of Na reacted to produced 2 moles of NaOH
Therefore,
1.91 moles of Na will also react to produce 1.91 moles of NaOH
Finally, we shall determine the volume of the 2.00 M NaOH produced. Details below:
Molarity of NaOH = 2.00 MMole of NaOH = 1.91 molesVolume of NaOH =?Volume = mole / molarity
Volume of NaOH = 1.91 / 2
Volume of NaOH = 0.955 L
Multiply by 1000 to express in milliliter
Volume of NaOH = 0.955 × 1000
Volume of NaOH = 955 mL
Learn more about volume:
https://brainly.com/question/29144710
#SPJ1
example 1: 0.4 gm of compound c was reacted with 3.0ml of compound d (2.8 m in ether) to give 0.5 gm of a product. identify the expected product and calculate the theoretical and percent yield.
Answers
The expected product in the reaction of compound C with compound D is unknown based on the information provided. To calculate the theoretical yield, we need the balanced chemical equation for the reaction. Without this information, we cannot determine the theoretical yield or calculate the percent yield.
However, if we had the balanced equation, we could use the given amounts of compound C and compound D to determine the limiting reactant and calculate the theoretical yield.
The percent yield is then calculated by dividing the actual yield (0.5 gm) by the theoretical yield and multiplying by 100. Without the necessary information, we cannot provide specific values for the theoretical or percent yield in this case.
To know more about compound visit:-
https://brainly.com/question/14117795
#SPJ11
A solution is 5.00% potassium chloride by mass. How much potassium chloride would you expect to collect by evaporating 150.0 g of the solution?

Answers
Answer:
C. 7.50g
Explanation:
The percent (%) by mass of a solute in a solution refers to the number of grams contained in 100g of solution by that solute. In this case, 5% by mass of pottasium chloride (KCl) means 5g of KCl is contained in 100g of solution.
Therefore, in 150g of solution, there would be:
5g/100g × 150g
= 0.05 × 150
= 7.50g of KCl solute.
Hence, 7.50g of pottasium chloride would be expected to be collected by evaporating 150.0 g of the solution.
Given the following data for the hydrate M
(NO
3
)
3
dot
×H
2
O, where M is a metal with the atomic mass 79.01 g/mol, Mass of Crucible and Lid 34.3317 Mass of Crucible, Lid and Hydrate 39.7109 Mass of Crucible, Lid and Anhydrous Salt (3rd Heating) 37.0499 What is the mass of hydrate, with correct significant figures? Your Answer: Answer units Given the following data for the hydrate M
(NO
3
)
3
dot
×H
2
O, where M is a metal with the atomic mass 78.37 g/mol, Mass of Crucible and Lid 34.2540 Mass of Crucible, Lid and Hydrate 39.1031 Mass of Crucible, Lid and Anhydrous Salt (3rd Heating) 37.3999 What was the mass of the anhydrous salt after the 3rd heating, with the correct significant figures? Your Answer:
Answers
The mass of the hydrate is 5.3792 grams and the mass of the anhydrous salt after the 3rd heating is 3.1459 grams.
A hydrate is a compound that contains a specific number of water molecules chemically bound to its structure. The formula of the hydrate is \(M(NO_3)_3_d_o_t_s * H_2O\), where M represents a metal.
A. Given information:
Mass of the crucible and lid (initial measurement): 34.3317 gMass of the crucible, lid, and hydrate (after adding the hydrate): 39.7109 gMass of the crucible, lid, and anhydrous salt (after heating to remove water): 37.0499 gTo determine the mass of the hydrate, subtract the mass of the crucible and lid from the mass of the crucible, lid, and hydrate:
Mass of hydrate = Mass of crucible, lid, and hydrate - Mass of crucible and lid
Mass of hydrate = 39.7109 g - 34.3317 g
Mass of hydrate = 5.3792 g
B. Given information:
Mass of the crucible and lid (initial measurement): 34.2540 gMass of the crucible, lid, and hydrate (after adding the hydrate): 39.1031gMass of the crucible, lid, and anhydrous salt (after heating to remove water): 37.3999gMass of anhydrous salt = Mass of crucible, lid, and anhydrous salt - Mass of crucible and lid.
Mass of anhydrous salt = 37.3999 g - 34.2540 g
Mass of anhydrous salt = 3.1459 g
Therefore, the mass of the hydrate is 5.3792 grams, and the mass of the anhydrous salt after the 3rd heating is 3.1459 grams.
To know more about Hydrant, click here:
https://brainly.com/question/31559603
#SPJ4
Complete question:
A. Given the following data for the hydrate \(M(NO_3)_3_d_o_t_s * H_2O\), where M is a metal with an atomic mass of 79.01 g/mol,
Mass of Crucible and Lid 34.3317 g
Mass of Crucible, Lid, and Hydrate 39.7109 g
Mass of Crucible, Lid, and Anhydrous Salt 37.0499 g
What is the mass of hydrate, with correct significant figures?
B. Given the following data for the hydrate \(M(NO_3)_3_d_o_t_s * H_2O\), where M is a metal with an atomic mass of 78.37 g/mol,
Mass of Crucible and Lid 34.2540 g
Mass of Crucible, Lid, and Hydrate 39.1031 g
Mass of Crucible, Lid, and Anhydrous Salt 37.3999g
What was the mass of the anhydrous salt after the \(3^r^d\) heating, with the correct significant figures?