Answers
Answer: The force on the firefly
The unfortunate firefly hitting the bus does not change the velocity of the bus very much. Technically there is a change, but it's so very small and miniscule that it barely registers. To any casual observer not paying very close attention, they don't notice anything at all. So effectively the force on the firefly is a lot greater since the firefly got the worst end of the deal.
So in short, we look at the velocity of each object and see which velocity changed the most. In this case, the firefly's velocity changed from whatever speed it was flying to 0 when it stops flying all together. That's why the force is greater on the bug.
Related Questions
QUESTION 54
What is/are the reactant(s) of the chemical reaction shown?
CH4 (g) + 202 (g) → CO2 (g) + 2H20 (g)
O CH4 only
O 02 only
O CH4 and 02
O CO2 and H20
The process above is not a chemical reaction
Answers
Answer:
the answer is C.
Explanation:
CH4 and 02
BRAINLLEST! Boiling water for mac and cheese is a physical change. Give 2 pieces of evidence to support this claim
Answers
Answer:
In explanation.
Explanation:
Physical changes are generally changes in states of matter. So, the water going from liquid to gas is a phase change and therefore physical. (It's still water, just in a different form). Im not sure about the second piece of evidence though. Unless you are talking about the Mac and Cheese which in that case is also a physical change as the two ingredients cheese and macaroni stay the same when cooked just become softer and edible.
Please help me with this question

Answers
Remember that
For being a bond covalent ∆E<1.8For being a bond ionic ∆E>1.8#1
∆EN=0.5Carbon is present so it's covalent
#2
OH bond is also covalent#3
P-H will hardly form a bond#4
Ionic bond#5
Ionic bond
What is true about a car with constant velocity?
A. It has a zero acceleration
B.It has a changing direction
C.postive acceleration
D. Negative acceleration
Answers
If something has a constant velocity, there is no acceleration!! Acceleration causes a change in a velocity and/or direction.
Answer:
Explanation:
c it’s see it
What is the hydroxide ion concentration of a solution with a pOH of 5.0?
Answers
Answer:
0.00001 M
Explanation:
The pOH of a solution is defined as the negative logarithm of the hydroxide ion concentration:
pOH = -log[OH-]
Therefore, we can rearrange this equation to solve for [OH-]:
[OH-] = 10^(-pOH)
If the pOH of a solution is 5.0, then:
[OH-] = 10^(-5.0)
[OH-] = 1.0 x 10^(-5.0)
[OH-] = 0.00001
Therefore, the hydroxide ion concentration of the solution is 0.00001 M.
Ocean water Choose one: A. is less dense than freshwater. B. salinity decreases as seawater evaporates. C. contains dissolved salts, like halite and gypsum. D. has an average salinity of 35%.
Answers
Answer: the answer is C
Explanation:
Ocean water contains dissolved salts, like halite and gypsum. The correct option is C.
What is the ocean?The ocean is the salty water that covers an area of 70.08% of the total surface. It is a large body of water that covers the entire earth. There are seven oceans present with different names, and they are connected with each other. The ocean is one of the traveling routes for traveling and exporting and import of products.
The average salinity of the ocean is 3.5%. It contains salts, halite, and gypsum-like minerals and substances.
Thus, the correct option is C. contains dissolved salts, like halite and gypsum.
Learn more about the ocean, it, here:
https://brainly.com/question/11803537
#SPJ2
Question 20 of 66
Which of the following species has the same number of protons as Fe3+?
A) Mn3+
B) Co2+
C) Fe2+
D) Nit
E) CO3+
Answers
If you have any questions please let me know.
Fe²⁺ has the same number of protons as Fe³⁺.
In order to know the number of protons of a species, we need to look for the atomic number (Z) in the Periodic Table.
The atomic number of Fe is 26, so it has 26 protons. Since protons are in the nucleus, they are not lost nor gained. Fe³⁺ results from Fe losing 3 electrons, but it has 26 protons as well.
Taking this into account, let's consider the number of protons of the following species:
A) Mn³⁺. It has 25 protons like Mn (Z = 25).
B) Co²⁺. It has 27 protons like Co (Z = 27).
C) Fe²⁺. It has 26 protons like Fe (Z = 26).
D) Ni. It has 28 protons (Z = 28).
E) Co³⁺. It has 27 protons like Co (Z = 27).
Fe²⁺ has the same number of protons as Fe³⁺.
You can learn more about atomic number here: https://brainly.com/question/17274608?referrer=searchResults

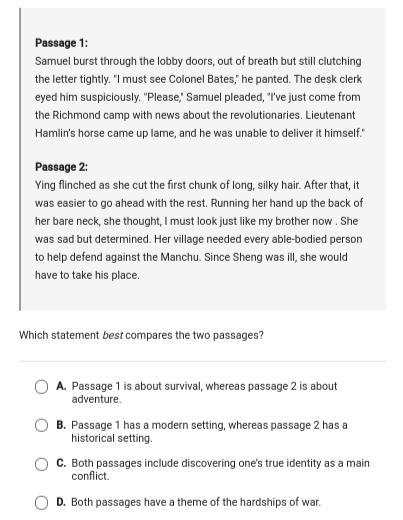
Semester Exams,Which Statement Best compares the two passages. Screenshot below,Giving Brainliest....
Answers
Combustion analysis is commonly used to determine the empirical formula of unknown compounds containing principally C and H. For a compound containing C, H, and O only, which of the following statements is INCORRECT?
Answers
"Combustion analysis can evaluate the molecular formula of unidentified compounds containing primarily C and H," is an incorrect statement.
Combustion analysis is a technique used to determine the amounts of carbon and hydrogen present in a compound by burning a sample and measuring the volume of gases produced. It can be used to determine the empirical formula of a compound containing C, H, and O only (which gives the ratio of atoms in a compound and not the actual number of atoms) but not the molecular formula (which gives the actual number of atoms in a compound). The molecular formula of a compound can be determined by other techniques like Mass Spectroscopy, IR spectroscopy, NMR spectroscopy etc.
To learn more about empirical formula here:
https://brainly.com/question/14044066
#SPJ4
Why does CO2 have a higher boiling point than CH4 when they both possess dispersion forces?
Answers
CO₂ have a higher boiling point than CH₄ when they both possess dispersion forces because CO₂ consists of polar bonds between carbon and oxygen.
Intermolecular forces determine bulk properties, such as the melting points of solids and the boiling points of liquids.
Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them together, thereby forming bubbles of vapor within the liquid.
CO₂ have a higher boiling point than CH₄ when they both possess dispersion forces because CO₂ consists of polar bonds between carbon and oxygen. The polarity increases the boiling point of the molecule.
Learn more about Intermolecular forces, here:
https://brainly.com/question/31797315
#SPJ1
help whats 2+2 i really don't know what the answer is i think it 1,250 but idk
Answers
Answer:
its 4
Explanation:
Answer:
4 is the answer
Explanation:
2 fingers plus 2 fingers is 4 fingers, Not 1,250
A small amount of salt dissolved in water is an example of a __________.
Answers
Answer: Homogeneous mixture
What is the length of the paper clip in cm?

Answers
Answer:
the paper clip is exactly 7.5 cm
Hi. Please tell me When you test starch with Barfoed’s reagent, what would be the answer, positive or negative? Explain the answer by giving reasons and structures.
Answers
The stream table shows the time needed for water to soak into the playfield soil.
Answers
Answer:
TRUE
Explanation:
plz brian list
Answer:
True
Explanation:
Calculate the percent ionization of benzoic acid for the following concentrations:
The ionization constant for benzoic acid is 6.25 x 10^-5
.00080 M : %
Answers
The concentration of H+ in the ionization is 0.00022 M. The initial concentration of the acid is 0.00080 M. Then , the percent ionization is 27.9 %.
What is percent ionization ?The percent ionization of an acid can be written as follows:
% ionization = [H+]eq /[HA]0 × 100
where [H+]eq is equilibrium concentration of H+ and /[HA]0 is the initial concentration of the acid.
Given , Ka of benzoic acid = [H +] [ph-]/[PhCOOH] =6.25 × 10⁻⁵
[PhCOOH] = 0.0008 M
[H +] = [ph-]
then, [H+²] = 0.0008 M × 6.25 × 10⁻⁵
[H +] = 0.00022 M.
Now, the percent ionization is calculated as follows:
% ionization = 0.00022 M / 0.00080 M × 100
= 27.9 %.
Therefor, the percent ionization of benzoic acid for the given concentration is 27.9 %.
Find more on percent ionization:
https://brainly.com/question/14225136
#SPJ1
Can someone help me I am stuck on this question it would mean the world if u helped me =) have a nice day!

Answers
Describing Dilutions
Which methods could be used to dilute a solution of sodium chloride (NaCl)? Select all that apply.
Add more NaCl to the solution.
Add more water to the solution.
Remove a small amount of the solution and mix it with water.
Heat the solution to evaporate some of the water.
Answers
To dilute a solution of sodium chloride (NaCl), we can use the following methods: Add more water to the solution and Remove a small amount of the solution and mix it with water.
By adding water, you increase the overall volume of the solution, which effectively reduces the concentration of NaCl. This is the most common and straightforward method of dilution.
By taking out a portion of the concentrated solution and mixing it with water, you decrease the concentration of NaCl in that portion, resulting in overall dilution when you combine it back with the rest of the solution.
By heating the solution, you cause the water to evaporate, leaving behind a more concentrated solution of NaCl. This process does not dilute the solution but rather concentrates it further.
Adding more NaCl to the solution would actually increase the concentration, not dilute it. Therefore, it is not a valid method for dilution.
learn more about Sodium chloride
https://brainly.com/question/30468270
As part of an investigation of the population of foxes on Sunday Gill island a scientist graphed the number of foxes presented on the island over a Spam of 15 years as shown below the study began with the earlier 0 and run until the start of year 15 According to the graph during the witch year the event reduced the carrying capacity of the area
Answers
The carrying capacity of the area was reduced in the year 10 according to the graph that shows the number of foxes on the island over a span of 15 years.
The graph shows a population of foxes over a span of 15 years. The y-axis represents the number of foxes on the island, while the x-axis represents time. The study began with the earlier 0 and ran until the start of year 15. According to the graph, the carrying capacity of the area was reduced in the year 10.
In the graph, it is shown that the population of foxes on Sunday Gill island had a significant increase from year 0 to year 3. After year 3, the fox population started to decrease and then remained fairly constant until year 10. After year 10, the population of foxes on the island started to decline more rapidly until the end of the study in year 15
This decline in the population of foxes on the island is most likely due to the reduction in carrying capacity of the area. Carrying capacity refers to the maximum number of individuals that an environment can sustain. When the carrying capacity of an environment is reached, it means that the environment can no longer provide the necessary resources to sustain the population.
There are various factors that can cause a reduction in carrying capacity, such as environmental degradation, competition for resources, or a natural disaster. In this case, it is not clear what caused the reduction in carrying capacity in year 10, but it is likely that it was due to some environmental factor that impacted the availability of resources for the fox population.
For more such information on: graph
https://brainly.com/question/31305548
#SPJ8
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
WORKSHEET- MOLECULAR AND IONIC EQUATIONSWrite molecular, total-ionic and net-ionic equations for the following below. (Any soluble substance givenbelow is an aqueous solution)

Answers
ANSWER
EXPLANATION
Given compound
\(\begin{gathered} \text{ Ammonium bromide }\rightarrow\text{ NH}_4Br \\ \text{ Sodium hydroxide }\rightarrow\text{ NaOH} \end{gathered}\)The next step is to write the molecular equation of the reaction between ammonium bromide and sodium hydroxide
\(\begin{gathered} \text{ Molecular equation of the reaction;} \\ \text{ NH}_4Br_{(aq)}\text{ + NaOH}_{(aq)}\rightarrow NaBr_{(aq)}\text{ + NH}_{3(aq)}\text{ + H}_2O_{(l)} \end{gathered}\)The next step is to write the total ionic equation.
To write the total ionic equation, follow the steps below
1. Break the compounds that exist in the aqueous state into ions
\(\begin{gathered} \text{ NH}_4Br\rightarrow\text{ NH}_4^++\text{ Br}^- \\ NaOH\text{ }\rightarrow\text{ Na}^+\text{ + OH}^- \\ NaBr\text{ }\rightarrow\text{ Na}^+\text{ + Br}^- \\ \end{gathered}\)2. Combine the ions together
\(\text{ Total ionic equation; NH}_4^+\text{ + Br}^-+\text{ Na}^++\text{ OH}^-\rightarrow\text{ Na}^++\text{ Br}^-+\text{ NH}_{3(aq)}\text{ + H}_2O\)The last step is to write the net ionic equation
To write the net ionic equation, you have to cancel ions that appear on the reactants sides, and the products side
\(\begin{gathered} \text{ NH}_4^++\cancel{Br^-}+\cancel{Na^+}+\text{ OH}^-\rightarrow\cancel{Na^+}+\cancel{Br^-}+NH_{3(aq)\text{ }}+\text{ H}_2O_{(l)} \\ \\ \text{ Net ionic equation : NH}_4^++\text{ OH}^-\rightarrow NH_{3(aq)\text{ }}+\text{ H}_2O(l) \end{gathered}\)PLEASEEE HELP!! I WILL MARK BRANLIEST IF CORRECT!!!!!!!!!
10. Which pair of elements would form a polar covalent bond together?
A. copper and carbon
B. sulfur and chlorine
C. oxygen and sodium
D. fluorine and fluorine
Answers
Answer: B. Sulfur and chlorine would form a polar covalent bond together.
Explanation:
Polar covalent bonds are formed when atoms with different electronegativities form a covalent bond. In a polar covalent bond, the electrons in the bond are shared unequally, with one atom being slightly more electronegative than the other and therefore attracting the shared electrons more strongly. This creates a small electric dipole in the molecule, with the more electronegative atom at the negative end and the less electronegative atom at the positive end.
Sulfur and chlorine have different electronegativities and therefore would form a polar covalent bond. Copper and carbon, oxygen and sodium, and fluorine and fluorine would not form polar covalent bonds because their electronegativities are either too similar or not compatible for covalent bonding.
Cr3+3e=Cr is that a reduction or oxidation
Answers
The chromium ions with a +3 oxidation state are reduced to chromium atoms with an oxidation state of 0.The reduction of Cr^3+ to Cr in this chemical equation is an example of a reduction reaction.
The chemical equation Cr^3+ + 3e^- = Cr represents the reduction of chromium ions (Cr^3+) to elemental chromium (Cr). In this reaction, the chromium ions gain three electrons to form neutral chromium atoms. Reduction reactions involve the gain of electrons and a decrease in the oxidation state of an element.
During the reduction process, the chromium ions are undergoing a change in their electronic configuration, gaining three electrons to achieve a stable configuration. This reduction reaction typically occurs in the presence of a reducing agent that donates electrons, allowing the chromium ions to be reduced. By gaining three electrons, the chromium ions are reduced to their elemental form, which has a neutral charge and an oxidation state of 0.
For more such questions on Reduction reaction
https://brainly.com/question/21851295
#SPJ8
All combustion reactions have oxygen as a reactant. (2 points)
Group of answer choices
True
False
Answers
Answer:
true
Explanation:
They all have a hydrocarbon plus oxygen.
The given statement that "all combustion reactions have oxygen as a reactant" is true. Combustion is a type of chemical reaction where a fuel (usually a hydrocarbon) combines with oxygen gas to produce heat, light, and new chemical compounds, such as carbon dioxide and water.
For combustion to occur, there must be a fuel source, oxygen, and a source of ignition, such as a spark or heat. Oxygen acts as a reactant because it combines with the fuel source to produce the new compounds. Without oxygen, the reaction cannot occur. It is important to note that not all reactions involving oxygen are combustion reactions. For example, rusting of iron is a reaction that involves oxygen, but it is not a combustion reaction.
In combustion reactions, the heat and light produced are often used for industrial processes, transportation, or heating. However, the reaction can also be destructive if not controlled, such as in wildfires or explosions. In conclusion, all combustion reactions have oxygen as a reactant, as it is necessary for the reaction to occur and produce the desired products.
For more such questions on combustion
https://brainly.com/question/13251946
#SPJ11
A propane tank when first filled reads 175. psi. After 1 month of use, the propane tank reads 81. psi.
Note: Reference the Conversion factors for non-SI units table for additional information.
Part 1 of 2
Convert the tank pressure when first filled to mmHg. Be sure your answer has the correct number of significant figures.
m
175. ps1=
mmHg
Answers
Answer:
After 1 month of use, the propane tank reads 81
Explanation:
Hurricanes and tropical storms gain their power from heated water evaporating from the ocean. Write a paragraph explaining why hurricanes typically weaken as they move into areas of cool water or over land.
Answers
As the disturbance gets to the land the warm ocean waters are no longer around thus the hurricane would die off rapidly
What is hurricane?The hurricane tends to occur when there is a rise of the warm air over the ocean. It should be noted that in the event of the hurricane there would be a sudden rising of the sea and this is going to rise so high that the violent waves would now begin to invade the land and cause a lot of destruction on its path as it is moving out from the ocean where the disturbance started.
Hence we can see that the origin of the hurricane is from some sort of a convection current that is taking place in the ocean and the hurricane must also be sustained by the existence of this convection current as the disturbance is moving away from the sea.
Learn more about Hurricane:https://brainly.com/question/13661501
#SPJ1
whoever answers my question correctly I'll give 50 points
Answers
Answer:
okii is it on your page
Explanation:
Answer:
hello
Explanation:
You have a basketball at a temperature of 298K, and a pressure of 2.3 atm. You leave it outside on a cold day, and the temperature of the gas drops to 273K. What is the pressure in the basketball assuming the volume does not change?
Answers
Answer:
The pressure in the basketball is approximately 0.00045329 atm after experiencing the temperature drop.
Explanation:
Assuming that the volume of the basketball does not change, we can use the ideal gas law to calculate the pressure in the basketball:
PV = nRT
Where P is the pressure, V is the volume, n is the amount of substance (in moles), R is the gas constant, and T is the temperature.
To find the pressure, we will first need to rearrange the formula to solve for P:
P = nRT / V
Plugging in the given values, we have:
P = nRT / V
P = (m * 1.6605393 × 10^(-24) moles) * (0.082057 mol/L-atm) * (273.15 K) / (0.225 L)
P = 0.00045329 atm
So the pressure in the basketball is approximately 0.00045329 atm after experiencing the temperature drop.
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
A small-sized woman has 6.4 x 10-3 moles of hemoglobin (molar mass of hemoglobin = 64, 456 g/mol) in her blood. Show your work
How many hemoglobin molecules are in this person’s body?
What is the mass (g) of hemoglobin molecules in this person’s body?
Answers
Answer:
Explanation:
Interesting question - especially if you are afflicted by something like Leukemia where it pays to know everything you can about Hemoglobin.
Molecules
1 mole of anything has 6.02 * 10^23 in this case molecules
6.4*10^-3 moles has x number of molecules
Set up the proportion
1/6.4*10^-3 = 6.02 * 10^23 molecule / x Cross Multiply
x = 6.4*10^-3 * 6.02 * 10^23
x = 3.853*10^21 molecules
Mass Hemoglobin
1 mol of hemoglobin is 64456 grams
6.4*10^-3 moles = x grams Cross multiply
x = 64456 * 6.4*10^-3
x = 412.5 grams of hemoglobin in this woman's body.
If you are American and think in pounds, then this is roughly a pound of hemoglobin.
Considering that you need hemoglobin to carry oxygen around to various organs, it is not an awful lot. That's the miracle of the human body.
